当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Allosteric Activation of Protein Phosphatase 5 with Small Molecules
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-08-15 , DOI: 10.1021/acs.jmedchem.4c00722 Qiuyue Zhang 1, 2 , Ling Yan 1, 2 , Lixiao Zhang 1, 2 , Jia Yu 1, 2 , Zeyu Han 1, 2 , Hua Liu 1, 2 , Jinying Gu 1, 2 , Keran Wang 1, 2 , Jiayi Wang 1, 2 , Fangsu Chen 1, 2 , Rongde Zhao 1, 2 , Yang Yan 1, 2 , Cheng Jiang 1, 2 , Qidong You 1, 2 , Lei Wang 1, 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-08-15 , DOI: 10.1021/acs.jmedchem.4c00722 Qiuyue Zhang 1, 2 , Ling Yan 1, 2 , Lixiao Zhang 1, 2 , Jia Yu 1, 2 , Zeyu Han 1, 2 , Hua Liu 1, 2 , Jinying Gu 1, 2 , Keran Wang 1, 2 , Jiayi Wang 1, 2 , Fangsu Chen 1, 2 , Rongde Zhao 1, 2 , Yang Yan 1, 2 , Cheng Jiang 1, 2 , Qidong You 1, 2 , Lei Wang 1, 2
Affiliation
|
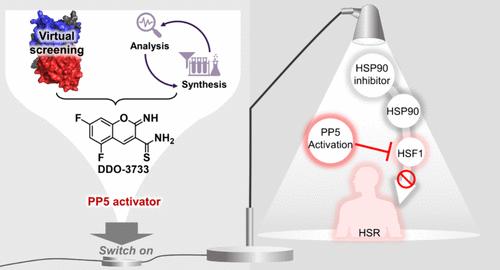
The activation of PP5 is essential for a variety of cellular processes, as it participates in a variety of biological pathways by dephosphorylating substrates. However, activation of PP5 by small molecules has been a challenge due to its native “self-inhibition” mechanism, which is controlled by the N-terminal TPR domain and the C-terminal αJ helix. Here, we reported the discovery of DDO-3733, a well-identified TPR-independent PP5 allosteric activator, which facilitates the dephosphorylation process of downstream substrates. Considering the negative regulatory effect of PP5 on heat shock transcription factor HSF1, pharmacologic activation of PP5 by DDO-3733 was found to reduce the HSP90 inhibitor-induced heat shock response. These results provide a chemical tool to advance the exploration of PP5 as a potential therapeutic target and highlight the value of pharmacological activation of PP5 to reduce heat shock toxicity of HSP90 inhibitors.
中文翻译:

小分子蛋白磷酸酶 5 的变构激活
PP5 的激活对于多种细胞过程至关重要,因为它通过底物去磷酸化参与多种生物途径。然而,由于PP5天然的“自我抑制”机制,由N端TPR结构域和C端αJ螺旋控制,小分子激活PP5一直是一个挑战。在这里,我们报告了DDO-3733的发现,这是一种经过充分鉴定的不依赖于 TPR 的 PP5 变构激活剂,可促进下游底物的去磷酸化过程。考虑到 PP5 对热休克转录因子 HSF1 的负调节作用,发现DDO-3733对 PP5 的药理激活可减少 HSP90 抑制剂诱导的热休克反应。这些结果为推进PP5作为潜在治疗靶点的探索提供了化学工具,并强调了PP5的药理学激活以降低HSP90抑制剂的热休克毒性的价值。
更新日期:2024-08-15
中文翻译:

小分子蛋白磷酸酶 5 的变构激活
PP5 的激活对于多种细胞过程至关重要,因为它通过底物去磷酸化参与多种生物途径。然而,由于PP5天然的“自我抑制”机制,由N端TPR结构域和C端αJ螺旋控制,小分子激活PP5一直是一个挑战。在这里,我们报告了DDO-3733的发现,这是一种经过充分鉴定的不依赖于 TPR 的 PP5 变构激活剂,可促进下游底物的去磷酸化过程。考虑到 PP5 对热休克转录因子 HSF1 的负调节作用,发现DDO-3733对 PP5 的药理激活可减少 HSP90 抑制剂诱导的热休克反应。这些结果为推进PP5作为潜在治疗靶点的探索提供了化学工具,并强调了PP5的药理学激活以降低HSP90抑制剂的热休克毒性的价值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号