Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactive Oxygen Species-Responsive Nanoparticle Delivery of Small Interfering Ribonucleic Acid Targeting Olfactory Receptor 2 for Atherosclerosis Theranostics
ACS Nano ( IF 15.8 ) Pub Date : 2024-08-14 , DOI: 10.1021/acsnano.4c07988
Huaner Ni 1, 2 , Hui Zhou 1 , Xin Liang 1 , Yulong Ge 2 , Hangwei Chen 2 , Junyi Liu 2 , Ben Wang 1 , Huiyu Chen 1 , Yujing Zhang 1 , Sihan Luo 1 , Ying Chen 1 , Xiaomei Lu 3, 4 , Chao Yin 1 , Quli Fan 1
ACS Nano ( IF 15.8 ) Pub Date : 2024-08-14 , DOI: 10.1021/acsnano.4c07988
Huaner Ni 1, 2 , Hui Zhou 1 , Xin Liang 1 , Yulong Ge 2 , Hangwei Chen 2 , Junyi Liu 2 , Ben Wang 1 , Huiyu Chen 1 , Yujing Zhang 1 , Sihan Luo 1 , Ying Chen 1 , Xiaomei Lu 3, 4 , Chao Yin 1 , Quli Fan 1
Affiliation
|
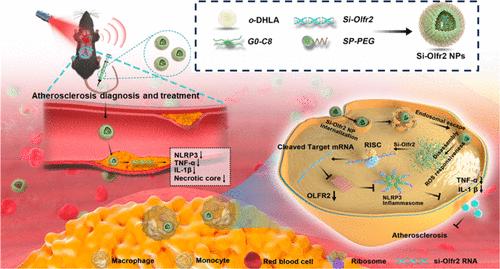
Atherosclerosis (AS) is a chronic inflammatory disorder characterized by arterial intimal lipid plaques. Small interfering ribonucleic acid (siRNA)-based therapies, with their ability to suppress specific genes with high targeting precision and minimal side effects, have shown great potential for AS treatment. However, targets of siRNA therapies based on macrophages for AS treatment are still limited. Olfactory receptor 2 (Olfr2), a potential target for plaque formation, was discovered recently. Herein, anti-Olfr2 siRNA (si-Olfr2) targeting macrophages was designed, and the theranostic platform encapsulating si-Olfr2 to target macrophages within atherosclerotic lesions was also developed, with the aim of downregulating Olfr2, as well as diagnosing AS through photoacoustic imaging (PAI) in the second near-infrared (NIR-II) window with high resolution. By utilization of a reactive oxygen species (ROS)-responsive nanocarrier system, the expression of Olfr2 on macrophages within atherosclerotic plaques was effectively downregulated, leading to the inhibition of NLR family pyrin domain containing 3 (NLRP3) inflammasome activation and interleukin-1 β (IL-1β) secretion, thereby reducing the formation of atherosclerotic plaques. As manifested by decreased Olfr2 expression, the lesions exhibited a significantly alleviated inflammatory response that led to reduced lipid deposition, macrophage apoptosis, and a noticeable decrease in the necrotic areas. This study provides a proof of concept for evaluating the theranostic nanoplatform to specifically deliver si-Olfr2 to lesional macrophages for AS diagnosis and treatment.
中文翻译:

靶向嗅觉受体 2 的小干扰核糖核酸的活性氧响应纳米颗粒递送治疗诊断学
动脉粥样硬化 (AS) 是一种以动脉内膜脂质斑块为特征的慢性炎症性疾病。基于小干扰核糖核酸 (siRNA) 的疗法能够以高靶向精度和最小的副作用抑制特定基因,在 AS 治疗中显示出巨大的潜力。然而,基于巨噬细胞的 siRNA 疗法用于 AS 治疗的靶点仍然有限。嗅觉受体 2 (Olfr2) 是斑块形成的潜在靶标,最近被发现。在此,设计了靶向巨噬细胞的抗 Olfr2 siRNA (si-Olfr2),还开发了封装 si-Olfr2 以靶向动脉粥样硬化病变内巨噬细胞的治疗诊断平台,目的是下调 Olfr2,以及通过光声成像 (PAI) 在第二个近红外 (NIR-II) 窗口中以高分辨率诊断 AS。通过利用活性氧 (ROS) 反应性纳米载体系统,动脉粥样硬化斑块内巨噬细胞上 Olfr2 的表达被有效下调,导致抑制 NLR 家族包含 3 (NLRP3) 炎性小体的 pyrin 结构域激活和白细胞介素-1 β (IL-1β) 分泌,从而减少动脉粥样硬化斑块的形成。如 Olfr2 表达降低所表现的那样,病变表现出炎症反应的显着缓解,导致脂质沉积减少、巨噬细胞凋亡和坏死区域的显着减少。本研究为评估治疗诊断纳米平台特异性将 si-Olfr2 递送至病变巨噬细胞用于 AS 诊断和治疗提供了概念验证。
更新日期:2024-08-14
中文翻译:

靶向嗅觉受体 2 的小干扰核糖核酸的活性氧响应纳米颗粒递送治疗诊断学
动脉粥样硬化 (AS) 是一种以动脉内膜脂质斑块为特征的慢性炎症性疾病。基于小干扰核糖核酸 (siRNA) 的疗法能够以高靶向精度和最小的副作用抑制特定基因,在 AS 治疗中显示出巨大的潜力。然而,基于巨噬细胞的 siRNA 疗法用于 AS 治疗的靶点仍然有限。嗅觉受体 2 (Olfr2) 是斑块形成的潜在靶标,最近被发现。在此,设计了靶向巨噬细胞的抗 Olfr2 siRNA (si-Olfr2),还开发了封装 si-Olfr2 以靶向动脉粥样硬化病变内巨噬细胞的治疗诊断平台,目的是下调 Olfr2,以及通过光声成像 (PAI) 在第二个近红外 (NIR-II) 窗口中以高分辨率诊断 AS。通过利用活性氧 (ROS) 反应性纳米载体系统,动脉粥样硬化斑块内巨噬细胞上 Olfr2 的表达被有效下调,导致抑制 NLR 家族包含 3 (NLRP3) 炎性小体的 pyrin 结构域激活和白细胞介素-1 β (IL-1β) 分泌,从而减少动脉粥样硬化斑块的形成。如 Olfr2 表达降低所表现的那样,病变表现出炎症反应的显着缓解,导致脂质沉积减少、巨噬细胞凋亡和坏死区域的显着减少。本研究为评估治疗诊断纳米平台特异性将 si-Olfr2 递送至病变巨噬细胞用于 AS 诊断和治疗提供了概念验证。

































 京公网安备 11010802027423号
京公网安备 11010802027423号