当前位置:
X-MOL 学术
›
Pharmacol. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cardiovascular Pharmacogenetics: From Discovery of Genetic Association to Clinical Adoption of Derived Test
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2024-09-01 , DOI: 10.1124/pharmrev.123.000750 Benoît Delabays 1 , Katerina Trajanoska 1 , Joshua Walonoski 1 , Vincent Mooser 2
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2024-09-01 , DOI: 10.1124/pharmrev.123.000750 Benoît Delabays 1 , Katerina Trajanoska 1 , Joshua Walonoski 1 , Vincent Mooser 2
Affiliation
|
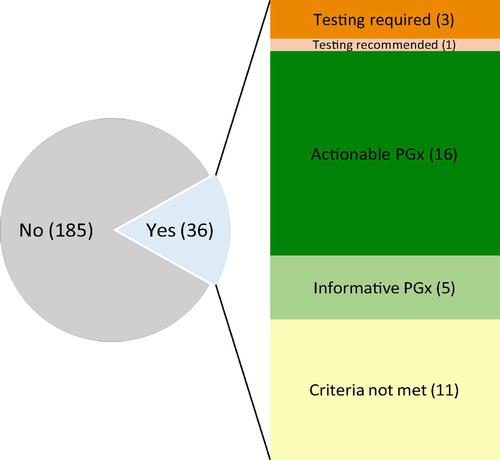
Recent breakthroughs in human genetics and in information technologies have markedly expanded our understanding at the molecular level of the response to drugs, i.e., pharmacogenetics (PGx), across therapy areas. This review is restricted to PGx for cardiovascular (CV) drugs. First, we examined the PGx information in the labels approved by regulatory agencies in Europe, Japan, and North America and related recommendations from expert panels. Out of 221 marketed CV drugs, 36 had PGx information in their labels approved by one or more agencies. The level of annotations and recommendations varied markedly between agencies and expert panels. Clopidogrel is the only CV drug with consistent PGx recommendation (i.e., “actionable”). This situation prompted us to dissect the steps from discovery of a PGx association to clinical translation. We found 101 genome-wide association studies that investigated the response to CV drugs or drug classes. These studies reported significant associations for 48 PGx traits mapping to 306 genes. Six of these 306 genes are mentioned in the corresponding PGx labels or recommendations for CV drugs. Genomic analyses also highlighted the wide between-population differences in risk allele frequencies and the individual load of actionable PGx variants. Given the high attrition rate and the long road to clinical translation, additional work is warranted to identify and validate PGx variants for more CV drugs across diverse populations and to demonstrate the utility of PGx testing. To that end, pre-emptive PGx combining genomic profiling with electronic medical records opens unprecedented opportunities to improve healthcare, for CV diseases and beyond.
中文翻译:

心血管药物遗传学:从遗传关联的发现到衍生测试的临床采用
人类遗传学和信息技术的最新突破显着扩展了我们对跨治疗领域药物反应的分子水平(即药物遗传学(PGx))的理解。本次审查仅限于心血管 (CV) 药物的 PGx。首先,我们检查了欧洲、日本和北美监管机构批准的标签中的 PGx 信息以及专家小组的相关建议。在 221 种上市的 CV 药物中,有 36 种在其标签中含有经一个或多个机构批准的 PGx 信息。各机构和专家小组之间的注释和建议水平存在显着差异。氯吡格雷是唯一具有一致 PGx 推荐(即“可操作”)的 CV 药物。这种情况促使我们剖析从发现 PGx 关联到临床转化的步骤。我们发现了 101 项全基因组关联研究,调查了对 CV 药物或药物类别的反应。这些研究报告了 48 个 PGx 性状与 306 个基因的显着关联。这 306 个基因中的 6 个在 CV 药物的相应 PGx 标签或建议中提到。基因组分析还强调了风险等位基因频率和可操作 PGx 变体的个体负载方面的人群之间的巨大差异。鉴于高流失率和漫长的临床转化之路,需要开展额外的工作来识别和验证不同人群中更多 CV 药物的 PGx 变体,并证明 PGx 测试的实用性。为此,先发制人的 PGx 将基因组分析与电子病历相结合,为改善心血管疾病及其他疾病的医疗保健提供了前所未有的机会。
更新日期:2024-08-15
中文翻译:

心血管药物遗传学:从遗传关联的发现到衍生测试的临床采用
人类遗传学和信息技术的最新突破显着扩展了我们对跨治疗领域药物反应的分子水平(即药物遗传学(PGx))的理解。本次审查仅限于心血管 (CV) 药物的 PGx。首先,我们检查了欧洲、日本和北美监管机构批准的标签中的 PGx 信息以及专家小组的相关建议。在 221 种上市的 CV 药物中,有 36 种在其标签中含有经一个或多个机构批准的 PGx 信息。各机构和专家小组之间的注释和建议水平存在显着差异。氯吡格雷是唯一具有一致 PGx 推荐(即“可操作”)的 CV 药物。这种情况促使我们剖析从发现 PGx 关联到临床转化的步骤。我们发现了 101 项全基因组关联研究,调查了对 CV 药物或药物类别的反应。这些研究报告了 48 个 PGx 性状与 306 个基因的显着关联。这 306 个基因中的 6 个在 CV 药物的相应 PGx 标签或建议中提到。基因组分析还强调了风险等位基因频率和可操作 PGx 变体的个体负载方面的人群之间的巨大差异。鉴于高流失率和漫长的临床转化之路,需要开展额外的工作来识别和验证不同人群中更多 CV 药物的 PGx 变体,并证明 PGx 测试的实用性。为此,先发制人的 PGx 将基因组分析与电子病历相结合,为改善心血管疾病及其他疾病的医疗保健提供了前所未有的机会。






























 京公网安备 11010802027423号
京公网安备 11010802027423号