Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-08-14 , DOI: 10.1002/adsc.202400760 Jiaming Xu 1 , Yanfeng Gao 1 , Xin Gao 2 , Zhiwei Miao 2
|
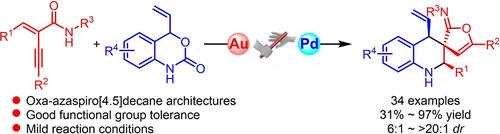
We present a diastereoselective Au/Pd relay catalytic tandem cyclization reaction to produce dearomatic 2-oxa-7-azaspiro[4.5]decane derivatives under mild conditions. This process involves generating furan-derived azadiene from readily available enynamide, followed by a [2+4] cycloaddition with Pd-π-allyl dipole decarboxylated from vinyl benzoxazinanone. Our method efficiently constructs the spiro[4.5]decane skeleton, achieving yields ranging from 31–97% and diastereoselectivities from 6:1 dr to >20:1 dr across 34 examples. This research introduces new cycloaddition sites for azadienes as 1,2-dipoles and offers a reliable approach for constructing oxa-azaspiro[4.5]decane frameworks.
中文翻译:

脱芳烃 2-氧杂-7-氮杂吡啶的非对映选择性合成[4.5]癸烷衍生物通过金和钯接力催化烯酰胺与乙烯基苯并恶氮杂氮酮的串联环化
我们提出了一种非对映选择性 Au/Pd 中继催化串联环化反应,在温和条件下产生脱芳烃 2-氧杂-7-氮杂[4.5]癸烷衍生物。该工艺包括从现成的烯酰胺中生成呋喃衍生的氮杂烯,然后用乙烯基苯并恶氮醌脱羧的 Pd-π-烯丙基偶极子进行 [2+4] 环加成反应。我们的方法有效地构建了螺[4.5]癸烷骨架,在 34 个实例中实现了 31-97% 的产率和非对映选择性从 6:1 dr 到 >20:1 dr。本研究引入了氮二烯作为 1,2-偶极子的新环加成位点,并为构建 oxa-azaspiro[4.5]癸烷框架提供了一种可靠的方法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号