当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent Advances in Scandium(III) Triflate Catalysis: A Review
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2024-08-14 , DOI: 10.1002/ajoc.202400295 Sumit Kumar 1 , Aditi Arora 1 , Shivani Sapra 1 , Riya Chaudhary 1 , Brajendra Kumar Singh 2 , Sunil K. Singh 3
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2024-08-14 , DOI: 10.1002/ajoc.202400295 Sumit Kumar 1 , Aditi Arora 1 , Shivani Sapra 1 , Riya Chaudhary 1 , Brajendra Kumar Singh 2 , Sunil K. Singh 3
Affiliation
|
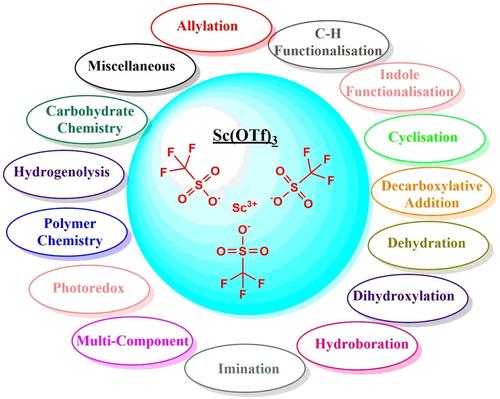
Over the past three decades, triflate salts have emerged as crucial Lewis acid catalysts in organic synthesis, playing a significant role in cyclization, C−H bond functionalization, and various other reactions. Among these, rare-earth triflates have garnered attention due to their water compatibility, environmental friendliness, noncorrosive nature, and reusability. In particular, scandium(III) triflate [Sc(OTf)3] stands out as a water-resistant Lewis acid with remarkable catalytic activity in aqueous environments. Unlike typical Lewis acids such as AlCl3, BF3, and SnCl4, which are decomposed or deactivated by water, Sc(OTf)3 remains stable and effective. Its exceptional Lewis acidity, resilience against hydrolysis, and recyclability make it a prominent green catalyst. The unique stability of Sc(OTf)3 in water is attributed to the smaller size of scandium ions (Sc3+), enhancing its catalytic efficiency. Sc(OTf)3 has a longstanding history in organic synthesis, facilitating a wide range of reactions including aldol, Michael, allylation, Friedel-Crafts acylations, Diels-Alder, Mannich, cycloadditions (including cyclopropanation), and cascade reactions. The increasing utilization of Sc(OTf)3 over the past decade underscores the necessity for updated insights. This review provides a concise overview of the versatility of Sc(OTf)3 as a catalyst, focusing on developments from 2017 to 2024.
中文翻译:

钪(III) 三氟甲磺酸催化研究进展
在过去的三十年里,三氟甲磺酸盐已成为有机合成中至关重要的路易斯酸催化剂,在环化、C-H 键官能化和各种其他反应中发挥着重要作用。其中,稀土三氟甲磺酸因其水相容性、环境友好性、无腐蚀性和可重复使用性而受到关注。特别是,三氟甲酸钪 [Sc(OTf)3] 是一种防水路易斯酸,在水性环境中具有显著的催化活性。与典型的路易斯酸(如 AlCl3、BF3 和 SnCl4)被水分解或失活不同,Sc(OTf)3 保持稳定和有效。其卓越的路易斯酸度、抗水解能力和可回收性使其成为一种突出的绿色催化剂。Sc(OTf)3 在水中的独特稳定性归因于钪离子 (Sc3+) 的较小尺寸,从而提高了其催化效率。Sc(OTf)3 在有机合成方面有着悠久的历史,可促进多种反应,包括羟醛、Michael、烯丙基化、Friedel-Crafts 酰化、Diels-Alder、Mannich、环加成反应(包括环丙烷化)和级联反应。在过去十年中,Sc(OTf)3 的利用率不断提高,这凸显了更新见解的必要性。本文简要概述了 Sc(OTf)3 作为催化剂的多功能性,重点介绍了 2017 年至 2024 年的发展情况。
更新日期:2024-08-14
中文翻译:

钪(III) 三氟甲磺酸催化研究进展
在过去的三十年里,三氟甲磺酸盐已成为有机合成中至关重要的路易斯酸催化剂,在环化、C-H 键官能化和各种其他反应中发挥着重要作用。其中,稀土三氟甲磺酸因其水相容性、环境友好性、无腐蚀性和可重复使用性而受到关注。特别是,三氟甲酸钪 [Sc(OTf)3] 是一种防水路易斯酸,在水性环境中具有显著的催化活性。与典型的路易斯酸(如 AlCl3、BF3 和 SnCl4)被水分解或失活不同,Sc(OTf)3 保持稳定和有效。其卓越的路易斯酸度、抗水解能力和可回收性使其成为一种突出的绿色催化剂。Sc(OTf)3 在水中的独特稳定性归因于钪离子 (Sc3+) 的较小尺寸,从而提高了其催化效率。Sc(OTf)3 在有机合成方面有着悠久的历史,可促进多种反应,包括羟醛、Michael、烯丙基化、Friedel-Crafts 酰化、Diels-Alder、Mannich、环加成反应(包括环丙烷化)和级联反应。在过去十年中,Sc(OTf)3 的利用率不断提高,这凸显了更新见解的必要性。本文简要概述了 Sc(OTf)3 作为催化剂的多功能性,重点介绍了 2017 年至 2024 年的发展情况。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号