当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Feasibility of Laboratory Equipment-Based Simulation Methods to Assess the Impact of Vehicle Transportation on Product Quality of mAb Dosing Solutions
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2024-08-14 , DOI: 10.1021/acs.molpharmaceut.4c00681 Kashappa Goud Desai 1 , Cait Sofa 1 , Ning Wang 1 , Bivash Mandal 1 , Brendan Blockus 1 , Nathan Heacock 1 , James D Colandene 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2024-08-14 , DOI: 10.1021/acs.molpharmaceut.4c00681 Kashappa Goud Desai 1 , Cait Sofa 1 , Ning Wang 1 , Bivash Mandal 1 , Brendan Blockus 1 , Nathan Heacock 1 , James D Colandene 1
Affiliation
|
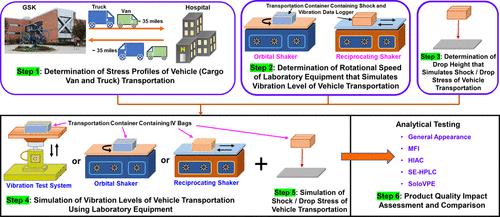
Therapeutic monoclonal antibody (mAb) products for intravenous (IV) administration generally require aseptic compounding with a commercially available diluent. When the administration site is located away from the preparation site, the prepared dosing solution may need to be transported in a vehicle. The impact of vehicle transportation on the product quality of mAbs needs to be evaluated to define safe handling and transportation conditions for dosing solutions. The design and execution of actual vehicle transportation studies require considerable resources and time. In this study, we systematically developed three different laboratory equipment-based methods that simulate vehicle transportation stresses: orbital shaker (OS), reciprocating shaker (RS), and vibration test system (VTS)-based simulation methods. We assessed their feasibility by comparing the impact on product quality caused by each simulated method with that caused by actual vehicle transportation. Without residual polysorbate 80 (PS80) in the mAb dosing solution, transportation via a cargo van led to a considerable increase in the subvisible particle counts and did not meet the compendial specifications for the light obscuration method. However, the presence of as low as 0.0004%w/v (4 ppm) PS80 in the dosing solution stabilized the mAb against vehicle transportation stresses and met the compendial specifications. Vehicle transportation of an IV bag with headspace resulted in negligible micro air bubbles and foaming in both PS80-free and PS80-containing mAb dosing solutions. These phenomena were found to be comparable to the VTS-based simulated method. However, the OS- and RS-based simulated methods formed significantly more micro air bubbles and foaming in an IV bag with headspace than either actual vehicle transportation or the VTS-based simulated method. Despite the higher interfacial stress (micro air bubbles and foaming) in the dosing solution created by the OS- and RS-based simulated methods, 0.0004%w/v (4 ppm) PS80 in the dosing solution was found to be sufficient to stabilize the mAb. The study shows that under appropriate simulated conditions, the OS-, RS-, and VTS-based simulated methods can be used as practical and meaningful models to assess the impact and risk of vehicle transportation on the quality of mAb dosing solutions.
中文翻译:

基于实验室设备的模拟方法评估车辆运输对单克隆抗体给药解决方案产品质量影响的可行性
用于静脉内 (IV) 给药的治疗性单克隆抗体 (mAb) 产品通常需要与市售稀释剂进行无菌混合。当给药地点远离制备地点时,制备好的给药溶液可能需要用车辆运输。需要评估车辆运输对单克隆抗体产品质量的影响,以确定给药解决方案的安全处理和运输条件。实际车辆运输研究的设计和执行需要大量的资源和时间。在这项研究中,我们系统地开发了三种不同的基于实验室设备的方法来模拟车辆运输应力:轨道振动台(OS)、往复振动台(RS)和基于振动测试系统(VTS)的模拟方法。我们通过比较每种模拟方法与实际车辆运输对产品质量的影响来评估其可行性。 mAb 给药溶液中没有残留聚山梨醇酯 80 (PS80),通过货车运输会导致亚可见颗粒计数显着增加,并且不符合光遮蔽方法的药典规范。然而,剂量溶液中低至 0.0004%w/v (4 ppm) PS80 的存在使 mAb 能够稳定地抵抗车辆运输压力,并符合药典规格。车辆运输带有顶空的静脉注射袋在不含 PS80 和含有 PS80 的 mAb 给药溶液中产生的微气泡和泡沫可以忽略不计。这些现象被发现与基于 VTS 的模拟方法相当。 然而,与实际车辆运输或基于 VTS 的模拟方法相比,基于 OS 和 RS 的模拟方法在具有顶部空间的 IV 袋中形成了明显更多的微气泡和泡沫。尽管基于 OS 和 RS 的模拟方法产生的配料溶液中存在较高的界面应力(微气泡和泡沫),但发现配料溶液中 0.0004%w/v (4 ppm) PS80 足以稳定单克隆抗体。研究表明,在适当的模拟条件下,基于OS、RS和VTS的模拟方法可以作为实用且有意义的模型来评估车辆运输对mAb给药方案质量的影响和风险。
更新日期:2024-08-14
中文翻译:

基于实验室设备的模拟方法评估车辆运输对单克隆抗体给药解决方案产品质量影响的可行性
用于静脉内 (IV) 给药的治疗性单克隆抗体 (mAb) 产品通常需要与市售稀释剂进行无菌混合。当给药地点远离制备地点时,制备好的给药溶液可能需要用车辆运输。需要评估车辆运输对单克隆抗体产品质量的影响,以确定给药解决方案的安全处理和运输条件。实际车辆运输研究的设计和执行需要大量的资源和时间。在这项研究中,我们系统地开发了三种不同的基于实验室设备的方法来模拟车辆运输应力:轨道振动台(OS)、往复振动台(RS)和基于振动测试系统(VTS)的模拟方法。我们通过比较每种模拟方法与实际车辆运输对产品质量的影响来评估其可行性。 mAb 给药溶液中没有残留聚山梨醇酯 80 (PS80),通过货车运输会导致亚可见颗粒计数显着增加,并且不符合光遮蔽方法的药典规范。然而,剂量溶液中低至 0.0004%w/v (4 ppm) PS80 的存在使 mAb 能够稳定地抵抗车辆运输压力,并符合药典规格。车辆运输带有顶空的静脉注射袋在不含 PS80 和含有 PS80 的 mAb 给药溶液中产生的微气泡和泡沫可以忽略不计。这些现象被发现与基于 VTS 的模拟方法相当。 然而,与实际车辆运输或基于 VTS 的模拟方法相比,基于 OS 和 RS 的模拟方法在具有顶部空间的 IV 袋中形成了明显更多的微气泡和泡沫。尽管基于 OS 和 RS 的模拟方法产生的配料溶液中存在较高的界面应力(微气泡和泡沫),但发现配料溶液中 0.0004%w/v (4 ppm) PS80 足以稳定单克隆抗体。研究表明,在适当的模拟条件下,基于OS、RS和VTS的模拟方法可以作为实用且有意义的模型来评估车辆运输对mAb给药方案质量的影响和风险。































 京公网安备 11010802027423号
京公网安备 11010802027423号