当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Recycling of Poly(cyclohexene carbonate)s via Synergistic Catalysis
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-08-12 , DOI: 10.1021/acsmacrolett.4c00372
Yan Yu 1 , Bai-Hao Ren 1 , Ye Liu 1 , Xiao-Bing Lu 1
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-08-12 , DOI: 10.1021/acsmacrolett.4c00372
Yan Yu 1 , Bai-Hao Ren 1 , Ye Liu 1 , Xiao-Bing Lu 1
Affiliation
|
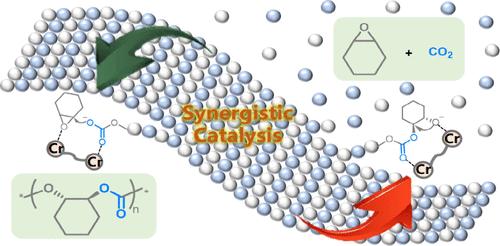
Chemical recycling of polymers to the corresponding monomers offers a valuable solution to address the current plastics crisis for creating an ideal and circular polymer economy. Here, we present a bimetallic synergistic depolymerization of the widely studied CO2-based polycarbonates, poly(cyclohexene carbonate)s, to epoxide monomers efficiently. The bimetallic CrIII-complex-mediated highly selective depolymerization and repolymerization was achieved via the regulation of reaction temperature, thus closing the circular loop of poly(cyclohexene carbonate)s in situ. Mechanistic investigation has revealed that the formation of epoxides undergoes a direct chain-end unzipping process. A bimetallic catalysis involving a nucleophilic attack of the metal-alkoxide species toward the methine carbon atom bound with an adjacent carbonyl that is activated by the other metal center features a lower energy barrier in DFT calculations, which promotes the epoxide extrusion.
中文翻译:

通过协同催化化学回收聚(环己烯碳酸酯)
将聚合物化学回收为相应的单体为解决当前的塑料危机、创建理想的循环聚合物经济提供了有价值的解决方案。在此,我们提出了广泛研究的CO 2基聚碳酸酯、聚(环己烯碳酸酯)的双金属协同解聚,有效地生成环氧化物单体。通过调节反应温度实现双金属Cr III配合物介导的高选择性解聚和再聚合,从而原位闭合聚环己烯碳酸酯的循环。机理研究表明,环氧化物的形成经历了直接的链端解链过程。双金属催化涉及金属醇盐物种对与相邻羰基结合的次甲基碳原子进行亲核攻击,该次甲基碳原子被另一个金属中心激活,在 DFT 计算中具有较低的能垒,从而促进环氧化物挤出。
更新日期:2024-08-12
中文翻译:

通过协同催化化学回收聚(环己烯碳酸酯)
将聚合物化学回收为相应的单体为解决当前的塑料危机、创建理想的循环聚合物经济提供了有价值的解决方案。在此,我们提出了广泛研究的CO 2基聚碳酸酯、聚(环己烯碳酸酯)的双金属协同解聚,有效地生成环氧化物单体。通过调节反应温度实现双金属Cr III配合物介导的高选择性解聚和再聚合,从而原位闭合聚环己烯碳酸酯的循环。机理研究表明,环氧化物的形成经历了直接的链端解链过程。双金属催化涉及金属醇盐物种对与相邻羰基结合的次甲基碳原子进行亲核攻击,该次甲基碳原子被另一个金属中心激活,在 DFT 计算中具有较低的能垒,从而促进环氧化物挤出。

































 京公网安备 11010802027423号
京公网安备 11010802027423号