当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enthalpic Classification of Water Molecules in Target–Ligand Binding
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-08-12 , DOI: 10.1021/acs.jcim.4c00794 Viktor Szél 1 , Balázs Zoltán Zsidó 1 , Csaba Hetényi 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-08-12 , DOI: 10.1021/acs.jcim.4c00794 Viktor Szél 1 , Balázs Zoltán Zsidó 1 , Csaba Hetényi 1
Affiliation
|
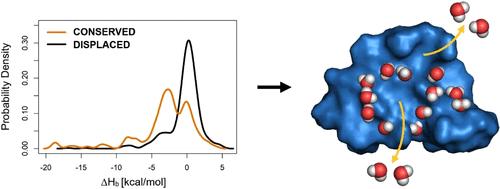
Water molecules play various roles in target–ligand binding. For example, they can be replaced by the ligand and leave the surface of the binding pocket or stay conserved in the interface and form bridges with the target. While experimental techniques supply target–ligand complex structures at an increasing rate, they often have limitations in the measurement of a detailed water structure. Moreover, measurements of binding thermodynamics cannot distinguish between the different roles of individual water molecules. However, such a distinction and classification of the role of individual water molecules would be key to their application in drug design at atomic resolution. In this study, we investigate a quantitative approach for the description of the role of water molecules during ligand binding. Starting from complete hydration structures of the free and ligand-bound target molecules, binding enthalpy scores are calculated for each water molecule using quantum mechanical calculations. A statistical evaluation showed that the scores can distinguish between conserved and displaced classes of water molecules. The classification system was calibrated and tested on more than 1000 individual water positions. The practical tests of the enthalpic classification included important cases of antiviral drug research on HIV-1 protease inhibitors and the Influenza A ion channel. The methodology of classification is based on open source program packages, Gromacs, Mopac, and MobyWat, freely available to the scientific community.
中文翻译:

目标-配体结合中水分子的焓分类
水分子在靶标-配体结合中发挥多种作用。例如,它们可以被配体取代并离开结合口袋的表面或在界面中保持保守并与靶标形成桥。虽然实验技术以越来越快的速度提供目标配体复合结构,但它们在测量详细的水结构方面通常存在局限性。此外,结合热力学的测量无法区分单个水分子的不同作用。然而,对单个水分子的作用进行这种区分和分类将是它们在原子分辨率药物设计中应用的关键。在这项研究中,我们研究了一种定量方法来描述水分子在配体结合过程中的作用。从游离和配体结合的目标分子的完整水合结构开始,使用量子力学计算计算每个水分子的结合焓分数。统计评估表明,这些分数可以区分保守的水分子和置换的水分子类别。该分类系统在 1000 多个单独的水位上进行了校准和测试。焓分类的实际测试包括HIV-1蛋白酶抑制剂和甲型流感离子通道等抗病毒药物研究的重要案例。分类方法基于开源程序包、Gromacs、Mopac 和 MobyWat,可供科学界免费使用。
更新日期:2024-08-12
中文翻译:

目标-配体结合中水分子的焓分类
水分子在靶标-配体结合中发挥多种作用。例如,它们可以被配体取代并离开结合口袋的表面或在界面中保持保守并与靶标形成桥。虽然实验技术以越来越快的速度提供目标配体复合结构,但它们在测量详细的水结构方面通常存在局限性。此外,结合热力学的测量无法区分单个水分子的不同作用。然而,对单个水分子的作用进行这种区分和分类将是它们在原子分辨率药物设计中应用的关键。在这项研究中,我们研究了一种定量方法来描述水分子在配体结合过程中的作用。从游离和配体结合的目标分子的完整水合结构开始,使用量子力学计算计算每个水分子的结合焓分数。统计评估表明,这些分数可以区分保守的水分子和置换的水分子类别。该分类系统在 1000 多个单独的水位上进行了校准和测试。焓分类的实际测试包括HIV-1蛋白酶抑制剂和甲型流感离子通道等抗病毒药物研究的重要案例。分类方法基于开源程序包、Gromacs、Mopac 和 MobyWat,可供科学界免费使用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号