当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic enantioselective (4+3) cyclization for the synthesis of spiro-fused heterocyclic compounds containing isoindolinone, oxepine and indole moieties
Chemical Communications ( IF 4.3 ) Pub Date : 2024-08-12 , DOI: 10.1039/d4cc03230f Kanghua Rui 1 , Shaoying Huang 1 , Yinong Wu 1 , Hanxiao Shen 1 , Xufeng Lin 1
Chemical Communications ( IF 4.3 ) Pub Date : 2024-08-12 , DOI: 10.1039/d4cc03230f Kanghua Rui 1 , Shaoying Huang 1 , Yinong Wu 1 , Hanxiao Shen 1 , Xufeng Lin 1
Affiliation
|
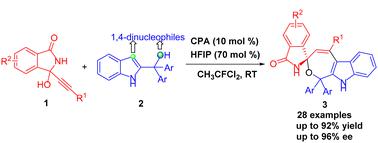
A refined strategy has been developed to control the regioselective asymmetric (4+3) cyclization of α-(3-isoindolinonyl) propargylic alcohols with 2-indolylmethanols, utilizing chiral phosphoric acid catalysis. This innovative organocatalytic cyclization yields spiro isoindolinone-oxepine-fused indoles featuring a spiro-quaternary stereocenter in high yields and enantioselectivities, facilitated by the solvent of 1,1-dichloro-1-fluoroethane and the additive of hexafluoroisopropanol. Additionally, the photophysical properties of product 3k are examined, revealing bright fluorescence both in the solution and the solid state.
中文翻译:

有机催化对映选择性 (4+3) 环化合成含有异吲哚酮、氧杂环庚烷和吲哚部分的螺稠杂环化合物
利用手性磷酸催化,开发了一种精细策略来控制 α-(3-异吲哚啉基) 炔丙醇与 2-吲哚甲醇的区域选择性不对称 (4+3) 环化。这种创新的有机催化环化反应在 1,1-二氯-1-氟乙烷溶剂和六氟异丙醇添加剂的促进下,以高产率和对映选择性产生具有螺环季立体中心的螺异吲哚酮-氧杂环戊烷稠合吲哚。此外,还检查了产品3k的光物理性质,显示出溶液和固态均发出明亮的荧光。
更新日期:2024-08-12
中文翻译:

有机催化对映选择性 (4+3) 环化合成含有异吲哚酮、氧杂环庚烷和吲哚部分的螺稠杂环化合物
利用手性磷酸催化,开发了一种精细策略来控制 α-(3-异吲哚啉基) 炔丙醇与 2-吲哚甲醇的区域选择性不对称 (4+3) 环化。这种创新的有机催化环化反应在 1,1-二氯-1-氟乙烷溶剂和六氟异丙醇添加剂的促进下,以高产率和对映选择性产生具有螺环季立体中心的螺异吲哚酮-氧杂环戊烷稠合吲哚。此外,还检查了产品3k的光物理性质,显示出溶液和固态均发出明亮的荧光。













































 京公网安备 11010802027423号
京公网安备 11010802027423号