当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative and Reductive Manipulation of C1 Resources by Bio-Inspired Molecular Catalysts to Produce Value-Added Chemicals
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-08-08 , DOI: 10.1021/acs.accounts.4c00390 Tomoya Ishizuka 1 , Takahiko Kojima 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-08-08 , DOI: 10.1021/acs.accounts.4c00390 Tomoya Ishizuka 1 , Takahiko Kojima 1
Affiliation
|
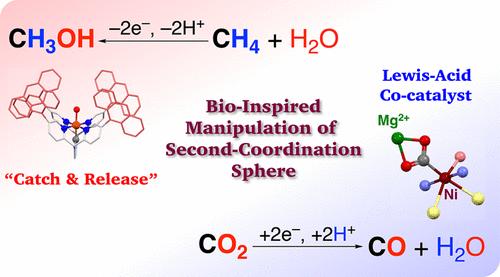
To tackle the energy and environmental concerns the world faces, much attention is given to catalytic reactions converting methane (CH4) and carbon dioxide (CO2) as abundant C1 resources into value-added chemicals with high efficiency and selectivity. In the oxidative conversion of CH4 to methanol, it is necessary to solve the requirement of strong oxidants due to the large bond-dissociation energy (BDE) of the C–H bonds in methane and achieve suppression of overoxidation due to the smaller BDE of the C–H bond in methanol as the product. On the other hand, to efficiently perform CO2 reduction, proton-coupled electron transfer (PCET) processes are required since the reduction potential of CO2 becomes positive by using proton-coupled processes; however, under the acidic conditions required for PCET, hydrogen evolution by the reduction of protons becomes competitive with CO2 reduction. Thus, it is indispensable to develop efficient catalysts for selective CO2 reduction. Recently, we have developed efficient catalytic reactions toward the alleviation of the concerns mentioned above. Concerning CH4 oxidation, inspired by metalloenzymes that oxidize hydrophobic organic substrates, a hydrophobic second coordination sphere (SCS) was introduced to an FeII complex bearing a pentadentate N-heterocyclic carbene ligand, and the FeII complex was used as a catalyst for CH4 oxidation in aqueous media. Consequently, CH4 was efficiently and selectively oxidized to methanol with 83% selectivity and a turnover number of 500. In contrast, when methanol was used as a substrate for catalytic oxidation by the FeII complex, oxidation products were obtained in a negligible yield, which was comparable to that of the control experiment without the catalyst. Therefore, the hydrophobic SCS of the FeII complex can capture only hydrophobic substrates such as CH4 and release hydrophilic products such as methanol to the aqueous medium for suppressing overoxidation (“catch-and-release” mechanism). On the other hand, for photocatalytic CO2 reduction, we have developed NiII complexes with N2S2-chelating ligands as catalysts, which have been inspired by carbon monoxide dehydrogenase, and have also introduced a binding site of Lewis-acidic metal ions to the SCS of the Ni complex. When Mg2+ was applied as a moderate Lewis acid, a Mg2+-bound Ni catalyst allowed us to achieve remarkable enhancement of the photocatalytic CO2 reduction to afford CO as the product with over 99% selectivity and a quantum yield of 11.4%. Divalent metal ions besides Mg2+ also showed similar positive impacts on photocatalytic CO2 reduction, whereas monovalent metal ions exhibited almost no effects and trivalent metal ions exclusively promoted hydrogen evolution. In this Account, we highlight our recent progress in the catalytic manipulations of CH4 and CO2 as C1 resources.
中文翻译:

通过仿生分子催化剂对 C1 资源进行氧化和还原操作来生产增值化学品
为了解决世界面临的能源和环境问题,将丰富的C1资源甲烷(CH 4 )和二氧化碳(CO 2 )转化为高效率和高选择性的增值化学品的催化反应受到广泛关注。在CH 4氧化转化为甲醇的过程中,需要解决由于甲烷中C-H键的键解离能(BDE)较大而需要强氧化剂的需求,并且由于BDE较小而实现抑制过氧化。甲醇中的C-H键作为产物。另一方面,为了有效地进行CO 2还原,需要质子耦合电子转移(PCET)工艺,因为通过使用质子耦合工艺,CO 2的还原电势变为正值;然而,在PCET所需的酸性条件下,通过质子还原产生的氢变得与CO 2还原竞争。因此,开发选择性CO 2还原的高效催化剂是必不可少的。最近,我们开发了有效的催化反应来缓解上述问题。关于CH 4氧化,受氧化疏水性有机底物的金属酶的启发,将疏水性第二配位球(SCS)引入到带有五齿N-杂环卡宾配体的Fe II配合物中,并将Fe II配合物用作CH 4 的催化剂4.在水介质中氧化。因此,CH 4被高效、选择性地氧化为甲醇,选择性为 83%,周转数为 500。 相反,当使用甲醇作为 Fe II配合物催化氧化的底物时,获得的氧化产物的产率可以忽略不计,这与没有催化剂的对照实验相当。因此,Fe II配合物的疏水性SCS只能捕获疏水性底物(例如CH 4 ) ,并将亲水性产物(例如甲醇)释放到水介质中以抑制过度氧化(“捕获和释放”机制)。另一方面,对于光催化CO 2还原,我们开发了具有N 2 S 2螯合配体作为催化剂的Ni II配合物,其受到一氧化碳脱氢酶的启发,并且还引入了路易斯酸性金属离子的结合位点到 Ni 络合物的 SCS。当Mg 2+用作温和的路易斯酸时,Mg 2+结合的Ni催化剂使我们能够显着增强光催化CO 2还原,以超过99%的选择性和11.4%的量子产率提供CO作为产物。除Mg 2+之外的二价金属离子也对光催化CO 2还原表现出类似的积极影响,而一价金属离子几乎没有表现出任何影响,而三价金属离子专门促进析氢。在本报告中,我们重点介绍了我们在 CH 4和 CO 2作为 C1 资源的催化操纵方面的最新进展。
更新日期:2024-08-08
中文翻译:

通过仿生分子催化剂对 C1 资源进行氧化和还原操作来生产增值化学品
为了解决世界面临的能源和环境问题,将丰富的C1资源甲烷(CH 4 )和二氧化碳(CO 2 )转化为高效率和高选择性的增值化学品的催化反应受到广泛关注。在CH 4氧化转化为甲醇的过程中,需要解决由于甲烷中C-H键的键解离能(BDE)较大而需要强氧化剂的需求,并且由于BDE较小而实现抑制过氧化。甲醇中的C-H键作为产物。另一方面,为了有效地进行CO 2还原,需要质子耦合电子转移(PCET)工艺,因为通过使用质子耦合工艺,CO 2的还原电势变为正值;然而,在PCET所需的酸性条件下,通过质子还原产生的氢变得与CO 2还原竞争。因此,开发选择性CO 2还原的高效催化剂是必不可少的。最近,我们开发了有效的催化反应来缓解上述问题。关于CH 4氧化,受氧化疏水性有机底物的金属酶的启发,将疏水性第二配位球(SCS)引入到带有五齿N-杂环卡宾配体的Fe II配合物中,并将Fe II配合物用作CH 4 的催化剂4.在水介质中氧化。因此,CH 4被高效、选择性地氧化为甲醇,选择性为 83%,周转数为 500。 相反,当使用甲醇作为 Fe II配合物催化氧化的底物时,获得的氧化产物的产率可以忽略不计,这与没有催化剂的对照实验相当。因此,Fe II配合物的疏水性SCS只能捕获疏水性底物(例如CH 4 ) ,并将亲水性产物(例如甲醇)释放到水介质中以抑制过度氧化(“捕获和释放”机制)。另一方面,对于光催化CO 2还原,我们开发了具有N 2 S 2螯合配体作为催化剂的Ni II配合物,其受到一氧化碳脱氢酶的启发,并且还引入了路易斯酸性金属离子的结合位点到 Ni 络合物的 SCS。当Mg 2+用作温和的路易斯酸时,Mg 2+结合的Ni催化剂使我们能够显着增强光催化CO 2还原,以超过99%的选择性和11.4%的量子产率提供CO作为产物。除Mg 2+之外的二价金属离子也对光催化CO 2还原表现出类似的积极影响,而一价金属离子几乎没有表现出任何影响,而三价金属离子专门促进析氢。在本报告中,我们重点介绍了我们在 CH 4和 CO 2作为 C1 资源的催化操纵方面的最新进展。































 京公网安备 11010802027423号
京公网安备 11010802027423号