当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Short-chain fatty acid butyrate against TMAO activating endoplasmic-reticulum stress and PERK/IRE1-axis with reducing atrial arrhythmia
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-08-05 , DOI: 10.1016/j.jare.2024.08.009 Tzu-Yu Cheng, Ting-Wei Lee, Shao-Jung Li, Ting-I Lee, Yao-Chang Chen, Yu-Hsun Kao, Satoshi Higa, Pao-Huan Chen, Yi-Jen Chen
中文翻译:

短链脂肪酸丁酸酯抗 TMAO 激活内质网应激和 PERK/IRE1 轴,减少房性心律失常
微生物群衍生的三甲胺 N-氧化物 (TMAO) 在心房中的积累与房性心律失常的发生和进展有关。丁酸盐是一种主要的短链脂肪酸,在维持肠道稳态和缓解全身炎症方面起着至关重要的作用,这可能会减少房性心律失常的发生。
本研究探讨了丁酸盐在调节 TMAO 介导的心房重塑和心律失常中的作用。
全细胞膜片钳实验、蛋白质印迹和免疫细胞化学分别用于分析 TMAO 处理的 HL-1 心房肌细胞在有或没有丁酸钠 (SB) 给药的情况下的电活性和信号传导。遥测心电图记录和超声心动图以及 Masson 三色染色和免疫组化分别用于检查接受 TMAO 治疗且有和没有 SB 给药的小鼠的心房功能和组织病理学。
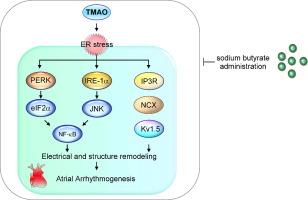
与对照细胞相比,TMAO 处理的 HL-1 心肌细胞表现出动作电位持续时间 (APD) 缩短、肌浆网 (SR) 钙含量升高、L 型钙电流 (ICa-L) 增加、Na + / Ca2 + 交换剂 (NCX) 电流增加和钾电流增加。然而,与对照组相比,SB 和 TMAO 的组合导致相似的 APD 、 SR 钙含量、 ICa-L 、瞬态向外钾电流 (Ito) 和超快速延迟整流钾电流 (IKur)。此外,与对照组或用 TMAO 和 SB 联合处理的 HL-1 细胞相比,TMAO 处理的 HL-1 心肌细胞表现出内质网 (ER) 应激信号的激活增加,以及 PKR 样 ER 应激激酶 (PERK)/IRE1α 轴激活增加和磷酸化 IP3R、NCX 和 Kv1.5 的表达。TMAO 处理的小鼠表现出心房异位搏动、心房功能受损、心房纤维化增加、 与对照组和用 TMAO 联合 SB 治疗的小鼠相比,PERK/IRE1α 轴激活对 ER 应激信号的激活更大。
TMAO 给药导致 PERK/IRE1α 轴激活,这可能会增加心房重塑和心律失常发生。SB 治疗减轻了 TMAO 诱发的 ER 应激。这一发现表明 SB 给药是治疗 TMAO 诱发的房性心律失常的有价值的策略。
更新日期:2024-08-05
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-08-05 , DOI: 10.1016/j.jare.2024.08.009 Tzu-Yu Cheng, Ting-Wei Lee, Shao-Jung Li, Ting-I Lee, Yao-Chang Chen, Yu-Hsun Kao, Satoshi Higa, Pao-Huan Chen, Yi-Jen Chen
|
Introduction
The accumulation of microbiota-derived trimethylamine N-oxide (TMAO) in the atrium is linked to the development and progression of atrial arrhythmia. Butyrate, a major short-chain fatty acid, plays a crucial role in sustaining intestinal homeostasis and alleviating systemic inflammation, which may reduce atrial arrhythmogenesis.Objectives
This study explored the roles of butyrate in regulating TMAO-mediated atrial remodeling and arrhythmia.Methods
Whole-cell patch clamp experiments, Western blotting, and immunocytochemistry were used to analyze electrical activity and signaling, respectively, in TMAO-treated HL-1 atrial myocytes with or without sodium butyrate (SB) administration. Telemetry electrocardiographic recording and echocardiography and Masson’s trichrome staining and immunohistochemistry were employed to examine atrial function and histopathology, respectively, in mice treated with TMAO with and without SB administration.Results
Compared with control cells, TMAO-treated HL-1 myocytes exhibited reduced action potential duration (APD), elevated sarcoplasmic reticulum (SR) calcium content, larger L-type calcium current (ICa-L), increased Na+/Ca2+ exchanger (NCX) current, and increased potassium current. However, the combination of SB and TMAO resulted in similar APD, SR calcium content, ICa-L, transient outward potassium current (Ito), and ultrarapid delayed rectifier potassium current (IKur) compared with controls. Additionally, TMAO-treated HL-1 myocytes exhibited increased activation of endoplasmic reticulum (ER) stress signaling, along with increased PKR-like ER stress kinase (PERK)/IRE1α axis activation and expression of phospho-IP3R, NCX, and Kv1.5, compared with controls or HL-1 cells treated with the combination of TMAO and SB. TMAO-treated mice exhibited atrial ectopic beats, impaired atrial function, increased atrial fibrosis, and greater activation of ER stress signaling with PERK/IRE1α axis activation compared with controls and mice treated with TMAO combined with SB.Conclusion
TMAO administration led to PERK/IRE1α axis activation, which may increase atrial remodeling and arrhythmogenesis. SB treatment mitigated TMAO-elicited ER stress. This finding suggests that SB administration is a valuable strategy for treating TMAO-induced atrial arrhythmia.中文翻译:

短链脂肪酸丁酸酯抗 TMAO 激活内质网应激和 PERK/IRE1 轴,减少房性心律失常
介绍
微生物群衍生的三甲胺 N-氧化物 (TMAO) 在心房中的积累与房性心律失常的发生和进展有关。丁酸盐是一种主要的短链脂肪酸,在维持肠道稳态和缓解全身炎症方面起着至关重要的作用,这可能会减少房性心律失常的发生。
目标
本研究探讨了丁酸盐在调节 TMAO 介导的心房重塑和心律失常中的作用。
方法
全细胞膜片钳实验、蛋白质印迹和免疫细胞化学分别用于分析 TMAO 处理的 HL-1 心房肌细胞在有或没有丁酸钠 (SB) 给药的情况下的电活性和信号传导。遥测心电图记录和超声心动图以及 Masson 三色染色和免疫组化分别用于检查接受 TMAO 治疗且有和没有 SB 给药的小鼠的心房功能和组织病理学。
结果
与对照细胞相比,TMAO 处理的 HL-1 心肌细胞表现出动作电位持续时间 (APD) 缩短、肌浆网 (SR) 钙含量升高、L 型钙电流 (ICa-L) 增加、Na + / Ca2 + 交换剂 (NCX) 电流增加和钾电流增加。然而,与对照组相比,SB 和 TMAO 的组合导致相似的 APD 、 SR 钙含量、 ICa-L 、瞬态向外钾电流 (Ito) 和超快速延迟整流钾电流 (IKur)。此外,与对照组或用 TMAO 和 SB 联合处理的 HL-1 细胞相比,TMAO 处理的 HL-1 心肌细胞表现出内质网 (ER) 应激信号的激活增加,以及 PKR 样 ER 应激激酶 (PERK)/IRE1α 轴激活增加和磷酸化 IP3R、NCX 和 Kv1.5 的表达。TMAO 处理的小鼠表现出心房异位搏动、心房功能受损、心房纤维化增加、 与对照组和用 TMAO 联合 SB 治疗的小鼠相比,PERK/IRE1α 轴激活对 ER 应激信号的激活更大。
结论
TMAO 给药导致 PERK/IRE1α 轴激活,这可能会增加心房重塑和心律失常发生。SB 治疗减轻了 TMAO 诱发的 ER 应激。这一发现表明 SB 给药是治疗 TMAO 诱发的房性心律失常的有价值的策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号