当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulating intermediate adsorption and H2O dissociation on a diatomic catalyst to promote electrocatalytic nitrate reduction to ammonia
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-08-07 , DOI: 10.1039/d4ee02747g Xiaoxue Zhang 1, 2 , Xiaokang Liu 1, 2 , Zhen-Feng Huang 1, 2, 3 , Li Gan 1, 2 , Shishi Zhang 1, 2 , Ru Jia 1, 2 , Muhammad Ajmal 1, 2 , Lun Pan 1, 2, 3 , Chengxiang Shi 1, 2, 3 , Xiangwen Zhang 1, 2, 3 , Guidong Yang 4 , Ji-Jun Zou 1, 2, 3
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-08-07 , DOI: 10.1039/d4ee02747g Xiaoxue Zhang 1, 2 , Xiaokang Liu 1, 2 , Zhen-Feng Huang 1, 2, 3 , Li Gan 1, 2 , Shishi Zhang 1, 2 , Ru Jia 1, 2 , Muhammad Ajmal 1, 2 , Lun Pan 1, 2, 3 , Chengxiang Shi 1, 2, 3 , Xiangwen Zhang 1, 2, 3 , Guidong Yang 4 , Ji-Jun Zou 1, 2, 3
Affiliation
|
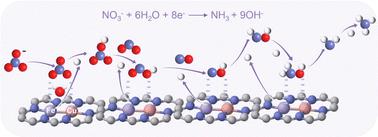
Electrochemical conversion of nitrate (NO3−) is an efficient approach to reduce NO3− pollutants and it offers a promising alternative for sustainable NH3 synthesis. However, this process is limited by the mismatched reaction kinetics of NO3− discharge, active hydrogen (H*) formation via water dissociation, and stepwise hydrogenation processes. Herein, using density functional theory (DFT) calculations, we screened a library of Cu–M diatomic catalysts coordinated with a N doped carbon matrix (Cu–M–N–C, M = Fe, Co, Ni, Mn, Zn) by balancing N-containing intermediate adsorption and H2O dissociation barriers. Among these catalysts, Cu–Fe–N–C demonstrates the best performance with a NH3 yield rate of 1.22 mmol h−1 cm−2 and a high Faradaic efficiency (FE) for NH3 synthesis of 95.08% at −0.8 V vs. the reversible hydrogen electrode, in which diatomic sites facilitate the first NO3− discharge step to generate adsorbed *NO3 and lower the energy barriers of the following hydrogenation/dehydration steps. More importantly, the incorporated Fe sites could promote the H2O dissociation, providing a large supply of H* for the deep hydrogenation of N-containing intermediates. This work reveals the tunable bonding interactions of diatomic sites with multiple reactant/intermediates, offering a new avenue for rational design of highly efficient atomic-level dispersed catalysts for both NO3− abatement and NH3 synthesis.
中文翻译:

调节双原子催化剂上的中间吸附和H2O解离促进电催化硝酸盐还原为氨
硝酸盐(NO 3 - )的电化学转化是减少NO 3 -污染物的有效方法,并且为可持续NH 3合成提供了一种有前景的替代方案。然而,该过程受到NO 3 -放电、通过水离解形成活性氢(H*) 以及逐步氢化过程的不匹配反应动力学的限制。在此,利用密度泛函理论(DFT)计算,我们筛选了与氮掺杂碳基体(Cu-M-N-C,M = Fe,Co,Ni,Mn,Zn)配位的Cu-M双原子催化剂库:平衡含氮中间吸附和H 2 O解离势垒。在这些催化剂中,Cu-Fe-N-C表现出最好的性能,NH 3产率为1.22 mmol h -1 cm -2 ,NH 3合成的法拉第效率(FE)在-0.8 V vs 95.08%可逆氢电极,其中双原子位点促进第一个NO 3 -放电步骤产生吸附的*NO 3并降低后续氢化/脱水步骤的能垒。 更重要的是,掺入的Fe位点可以促进H 2 O解离,为含氮中间体的深度氢化提供大量的H*。这项工作揭示了双原子位点与多种反应物/中间体的可调节键合相互作用,为合理设计用于NO 3 −减排和NH 3合成的高效原子级分散催化剂提供了新途径。
更新日期:2024-08-07
中文翻译:

调节双原子催化剂上的中间吸附和H2O解离促进电催化硝酸盐还原为氨
硝酸盐(NO 3 - )的电化学转化是减少NO 3 -污染物的有效方法,并且为可持续NH 3合成提供了一种有前景的替代方案。然而,该过程受到NO 3 -放电、通过水离解形成活性氢(H*) 以及逐步氢化过程的不匹配反应动力学的限制。在此,利用密度泛函理论(DFT)计算,我们筛选了与氮掺杂碳基体(Cu-M-N-C,M = Fe,Co,Ni,Mn,Zn)配位的Cu-M双原子催化剂库:平衡含氮中间吸附和H 2 O解离势垒。在这些催化剂中,Cu-Fe-N-C表现出最好的性能,NH 3产率为1.22 mmol h -1 cm -2 ,NH 3合成的法拉第效率(FE)在-0.8 V vs 95.08%可逆氢电极,其中双原子位点促进第一个NO 3 -放电步骤产生吸附的*NO 3并降低后续氢化/脱水步骤的能垒。 更重要的是,掺入的Fe位点可以促进H 2 O解离,为含氮中间体的深度氢化提供大量的H*。这项工作揭示了双原子位点与多种反应物/中间体的可调节键合相互作用,为合理设计用于NO 3 −减排和NH 3合成的高效原子级分散催化剂提供了新途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号