Cell Chemical Biology ( IF 6.6 ) Pub Date : 2024-08-07 , DOI: 10.1016/j.chembiol.2024.07.005 Kanak Raina 1 , Chris D Forbes 1 , Rebecca Stronk 1 , Jonathan P Rappi 1 , Kyle J Eastman 1 , Nilesh Zaware 1 , Xinheng Yu 1 , Hao Li 1 , Amit Bhardwaj 1 , Samuel W Gerritz 1 , Mia Forgione 1 , Abigail Hundt 1 , Madeline P King 1 , Zoe M Posner 1 , Allison D Correia 1 , Andrew McGovern 1 , David E Puleo 1 , Rebekka Chenard 1 , James J Mousseau 1 , J Ignacio Vergara 2 , Ethan Garvin 1 , Jennifer Macaluso 1 , Michael Martin 1 , Kyle Bassoli 1 , Kelli Jones 1 , Marco Garcia 1 , Katia Howard 1 , Madeleine Yaggi 1 , Levi M Smith 1 , Jinshan M Chen 1 , Andrew B Mayfield 2 , Cesar A De Leon 2 , John Hines 2 , Katherine J Kayser-Bricker 1 , Craig M Crews 3
|
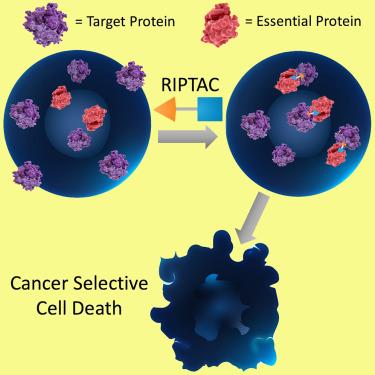
We describe a protein proximity inducing therapeutic modality called Regulated Induced Proximity Targeting Chimeras or RIPTACs: heterobifunctional small molecules that elicit a stable ternary complex between a target protein (TP) selectively expressed in tumor cells and a pan-expressed protein essential for cell survival. The resulting co-operative protein-protein interaction (PPI) abrogates the function of the essential protein, thus leading to death selectively in cells expressing the TP. This approach leverages differentially expressed intracellular proteins as novel cancer targets, with the advantage of not requiring the target to be a disease driver. In this chemical biology study, we design RIPTACs that incorporate a ligand against a model TP connected via a linker to effector ligands such as JQ1 (BRD4) or BI2536 (PLK1) or CDK inhibitors such as TMX3013 or dinaciclib. RIPTACs accumulate selectively in cells expressing the HaloTag-FKBP target, form co-operative intracellular ternary complexes, and induce an anti-proliferative response in target-expressing cells.
中文翻译:

调节诱导邻近靶向嵌合体 — RIPTAC — 一种用于癌症选择性治疗的异双功能小分子策略
我们描述了一种称为调节诱导接近靶向嵌合体或 RIPTAC 的蛋白质邻近诱导治疗方式:异双功能小分子,在肿瘤细胞中选择性表达的靶蛋白 (TP) 和细胞存活所必需的泛表达蛋白之间引发稳定的三元复合物。由此产生的协同蛋白-蛋白相互作用 (PPI) 消除了必需蛋白的功能,从而导致表达 TP 的细胞选择性死亡。这种方法利用差异表达的细胞内蛋白作为新的癌症靶点,其优点是不需要靶点成为疾病驱动因素。在这项化学生物学研究中,我们设计了 RIPTACs,其结合的针对模型 TP 的配体通过接头连接到效应配体,如 JQ1 (BRD4) 或 BI2536 (PLK1) 或 CDK 抑制剂,如 TMX3013 或 dinaciclib。RIPTAC 在表达 HaloTag-FKBP 靶标的细胞中选择性积累,形成协同的细胞内三元复合物,并在表达靶标的细胞中诱导抗增殖反应。































 京公网安备 11010802027423号
京公网安备 11010802027423号