当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photochemical [2 + 2] Cycloaddition Enables the Synthesis of Highly Thermally Stable and Acid/Base-Resistant Polyesters from a Nonpolymerizable α,β-Conjugated Valerolactone
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-08-05 , DOI: 10.1021/acsmacrolett.4c00398 Chun Yang 1 , Xiao-Tong Wu 1 , Lefei Yu 1 , Cheng-Ao Bi 1 , Fu-Sheng Du 1 , Zi-Chen Li 1
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-08-05 , DOI: 10.1021/acsmacrolett.4c00398 Chun Yang 1 , Xiao-Tong Wu 1 , Lefei Yu 1 , Cheng-Ao Bi 1 , Fu-Sheng Du 1 , Zi-Chen Li 1
Affiliation
|
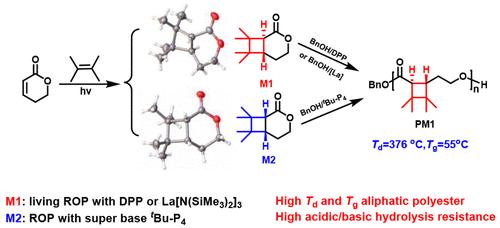
We report a simple strategy to transform a nonpolymerizable six-membered α,β-conjugated lactone, 5,6-dihydro-2H-pyran-2-one (DPO), into polymerizable bicyclic lactones via photochemical [2 + 2] cycloaddition. Two bicyclic lactones, M1 and M2, were obtained by the photochemical [2 + 2] cycloaddition of tetramethylethylene and DPO. Ring-opening polymerization (ROP) of M1 and M2 catalyzed by diphenyl phosphate (DPP), La[N(SiMe3)2]3, and 1-tert-butyl-4,4,4-tris(dimethylamino)-2,2-bis[tris (dimethylamino) phosphoranylide-namino]-2λ5, 4λ5-catenadi(phosphazene) (tBu-P4) were conducted. M1 is highly polymerizable, either DPP or La[N(SiMe3)2]3 could catalyze its living ROP under mild conditions, affording the well-defined PM1 with a predictable molar mass and low dispersity. M2 could only be polymerized with tBu-P4 as the catalyst, also generating the same polymer PM1. PM1 has high thermal stability, with a Td,5% being up to 376 °C. Ring-opening copolymerization (ROcP) of M1 and δ-valerolactone (δ-VL) catalyzed by La[N(SiMe3)2]3 afforded a series of random copolymers with enhanced thermal stabilities. Both PM1 and the copolymer containing 10 mol % M1 exhibited excellent resistance to acidic and basic hydrolysis. Our results demonstrate that direct photochemical [2 + 2] cycloaddition of α,β-conjugated valerolactone is not only a strategy to tune its polymerizability, but also allows for the synthesis of highly thermally stable aliphatic polyesters, inaccessible by other methods.
中文翻译:

光化学 [2 + 2] 环加成能够从不可聚合的 α,β-共轭戊内酯合成高度热稳定性和耐酸/碱聚酯
我们报告了一种简单的策略,通过光化学[2 + 2]环加成将不可聚合的六元α,β-共轭内酯5,6-二氢-2H-吡喃-2-酮( DPO )转化为可聚合的双环内酯。四甲基乙烯与DPO通过光化学[2+2]环加成反应得到两种双环内酯M1和M2 。 M1和M2在磷酸二苯酯(DPP)、La[N(SiMe 3 ) 2 ] 3和1-叔丁基-4,4,4-三(二甲氨基)-2的催化下进行开环聚合(ROP),进行2-双[三(二甲氨基)磷酰基-氨基]-2λ 5 , 4λ 5 -连环二(磷腈) ( t Bu-P 4 )。 M1具有高度聚合性,无论是DPP还是La[N(SiMe 3 ) 2 ] 3都可以在温和条件下催化其活性ROP,从而提供具有可预测摩尔质量和低分散度的明确PM1 。 M2只能以t Bu-P 4为催化剂聚合,也生成相同的聚合物PM1 。 PM1具有较高的热稳定性, T d,5%高达376℃。 La[N(SiMe 3 ) 2 ] 3催化M1和δ-戊内酯(δ-VL)的开环共聚(ROcP)得到一系列具有增强热稳定性的无规共聚物。 PM1和含有10mol% M1的共聚物均表现出优异的耐酸性和碱性水解性。 我们的结果表明,α,β-共轭戊内酯的直接光化学[2 + 2]环加成不仅是调节其聚合性的策略,而且还可以合成其他方法无法实现的高度热稳定的脂肪族聚酯。
更新日期:2024-08-05
中文翻译:

光化学 [2 + 2] 环加成能够从不可聚合的 α,β-共轭戊内酯合成高度热稳定性和耐酸/碱聚酯
我们报告了一种简单的策略,通过光化学[2 + 2]环加成将不可聚合的六元α,β-共轭内酯5,6-二氢-2H-吡喃-2-酮( DPO )转化为可聚合的双环内酯。四甲基乙烯与DPO通过光化学[2+2]环加成反应得到两种双环内酯M1和M2 。 M1和M2在磷酸二苯酯(DPP)、La[N(SiMe 3 ) 2 ] 3和1-叔丁基-4,4,4-三(二甲氨基)-2的催化下进行开环聚合(ROP),进行2-双[三(二甲氨基)磷酰基-氨基]-2λ 5 , 4λ 5 -连环二(磷腈) ( t Bu-P 4 )。 M1具有高度聚合性,无论是DPP还是La[N(SiMe 3 ) 2 ] 3都可以在温和条件下催化其活性ROP,从而提供具有可预测摩尔质量和低分散度的明确PM1 。 M2只能以t Bu-P 4为催化剂聚合,也生成相同的聚合物PM1 。 PM1具有较高的热稳定性, T d,5%高达376℃。 La[N(SiMe 3 ) 2 ] 3催化M1和δ-戊内酯(δ-VL)的开环共聚(ROcP)得到一系列具有增强热稳定性的无规共聚物。 PM1和含有10mol% M1的共聚物均表现出优异的耐酸性和碱性水解性。 我们的结果表明,α,β-共轭戊内酯的直接光化学[2 + 2]环加成不仅是调节其聚合性的策略,而且还可以合成其他方法无法实现的高度热稳定的脂肪族聚酯。































 京公网安备 11010802027423号
京公网安备 11010802027423号