当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design of Cr-Based Molecular Electrocatalyst Systems for the CO2 Reduction Reaction
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-08-06 , DOI: 10.1021/acs.accounts.4c00283 Megan E Moberg 1 , Charles W Machan 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-08-06 , DOI: 10.1021/acs.accounts.4c00283 Megan E Moberg 1 , Charles W Machan 1
Affiliation
|
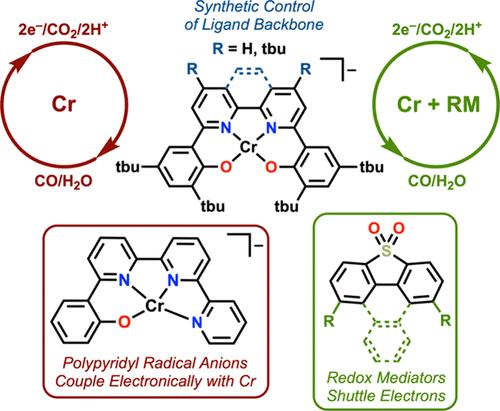
Human influence on the climate system was recently summarized by the sixth Intergovernmental Panel on Climate Change (IPCC) Assessment Report, which noted that global surface temperatures have increased more rapidly in the last 50 years than in any other 50-year period in the last 2000 years. Elevated global surface temperatures have had detrimental impacts, including more frequent and intense extreme weather patterns like flooding, wildfires, and droughts. In order to limit greenhouse gas emissions, various climate change policies, like emissions trading schemes and carbon taxes, have been implemented in many countries. The most prevalent anthropogenic greenhouse gas emitted is carbon dioxide (CO2), which accounted for 80% of all U.S. greenhouse gas emissions in 2022. The reduction of CO2 through the use of homogeneous electrocatalysts generally follows a two-electron/two-proton pathway to produce either carbon monoxide (CO) with water (H2O) as a coproduct or formic acid (HCOOH). These reduced carbon species are relevant to industrial applications: the Fischer–Tropsch process uses CO and H2 to produce fuels and commodity chemicals, while HCOOH is an energy dense carrier for fuel cells and useful synthetic reagent. Electrochemically reducing CO2 to value-added products is a potential way to address its steadily increasing atmospheric concentrations while supplanting the use of nonrenewable petrochemical reserves through the generation of new carbon-based resources. The selective electrochemical reduction of CO2 (CO2RR) by homogeneous catalyst systems was initially achieved with late (and sometimes costly) transition metal active sites, leading the field to conclude that transition metal complexes based on metals earlier in the periodic table, like chromium (Cr), were nonprivileged for the CO2RR. However, metals early in the table have sufficient reducing power to mediate the CO2RR and therefore could be selective in the correct coordination environment. This Account describes our efforts to develop and optimize novel Cr-based CO2RR catalyst systems through redox-active ligand modification strategies and the use of redox mediators (RMs). RMs are redox-active molecules which can participate cocatalytically during an electrochemical reaction, transferring electrons─often accompanied by protons─to a catalytic active site. Through mechanistic and computational work, we have found that ligand-based redox activity is key to controlling the intrinsic selectivity of these Cr compounds for CO2 activation. Ligand-based redox activity is also essential for developing cocatalytic systems, since it enables through-space interactions with reduced RMs containing redox-active planar aromatic groups, allowing charge transfer to occur within the catalyst assembly. Following a summary of our work, we offer a perspective on the possibilities for future development of catalytic and cocatalytic systems with early transition metals for small molecule activation.
中文翻译:

CO2 还原反应的铬基分子电催化剂体系设计
政府间气候变化专门委员会 (IPCC) 第六次评估报告最近总结了人类对气候系统的影响,该报告指出,过去 50 年全球地表温度的上升速度比过去 2000 年任何其他 50 年期间都要快年。全球地表温度升高产生了不利影响,包括洪水、野火和干旱等极端天气模式更加频繁和强烈。为了限制温室气体排放,许多国家实施了排放交易计划和碳税等各种气候变化政策。最普遍的人为温室气体排放是二氧化碳 (CO 2 ),到 2022 年,它占美国所有温室气体排放量的 80%。通过使用均相电催化剂减少 CO 2通常遵循双电子/双质子生产一氧化碳 (CO) 和水 (H 2 O) 作为副产品或甲酸 (HCOOH) 的途径。这些减少的碳物质与工业应用相关:费托工艺使用CO和H 2来生产燃料和商品化学品,而HCOOH是燃料电池和有用的合成试剂的能量密集载体。通过电化学方法将CO 2还原为增值产品是解决大气浓度稳步上升问题的一种潜在方法,同时通过生成新的碳基资源来取代不可再生石化储备的使用。 通过均相催化剂系统选择性电化学还原 CO 2 (CO 2 RR) 最初是通过后期(有时是昂贵的)过渡金属活性位点实现的,导致该领域得出结论,过渡金属配合物基于元素周期表中较早的金属,例如铬(Cr) 不享有CO 2 RR 的特权。然而,表中靠前的金属具有足够的还原能力来介导CO 2 RR,因此在正确的配位环境中可以具有选择性。本报告描述了我们通过氧化还原活性配体修饰策略和氧化还原介体 (RM) 的使用来开发和优化新型 Cr 基 CO 2 RR 催化剂系统的努力。 RM 是氧化还原活性分子,可以在电化学反应过程中起辅助催化作用,将电子(通常伴随着质子)转移到催化活性位点。通过机械和计算工作,我们发现基于配体的氧化还原活性是控制这些 Cr 化合物对 CO 2活化的内在选择性的关键。基于配体的氧化还原活性对于开发共催化系统也至关重要,因为它能够与含有氧化还原活性平面芳族基团的还原性 RM 进行空间相互作用,从而允许在催化剂组件内发生电荷转移。在总结我们的工作之后,我们对未来开发用于小分子活化的早期过渡金属催化和助催化系统的可能性提出了展望。
更新日期:2024-08-06
中文翻译:

CO2 还原反应的铬基分子电催化剂体系设计
政府间气候变化专门委员会 (IPCC) 第六次评估报告最近总结了人类对气候系统的影响,该报告指出,过去 50 年全球地表温度的上升速度比过去 2000 年任何其他 50 年期间都要快年。全球地表温度升高产生了不利影响,包括洪水、野火和干旱等极端天气模式更加频繁和强烈。为了限制温室气体排放,许多国家实施了排放交易计划和碳税等各种气候变化政策。最普遍的人为温室气体排放是二氧化碳 (CO 2 ),到 2022 年,它占美国所有温室气体排放量的 80%。通过使用均相电催化剂减少 CO 2通常遵循双电子/双质子生产一氧化碳 (CO) 和水 (H 2 O) 作为副产品或甲酸 (HCOOH) 的途径。这些减少的碳物质与工业应用相关:费托工艺使用CO和H 2来生产燃料和商品化学品,而HCOOH是燃料电池和有用的合成试剂的能量密集载体。通过电化学方法将CO 2还原为增值产品是解决大气浓度稳步上升问题的一种潜在方法,同时通过生成新的碳基资源来取代不可再生石化储备的使用。 通过均相催化剂系统选择性电化学还原 CO 2 (CO 2 RR) 最初是通过后期(有时是昂贵的)过渡金属活性位点实现的,导致该领域得出结论,过渡金属配合物基于元素周期表中较早的金属,例如铬(Cr) 不享有CO 2 RR 的特权。然而,表中靠前的金属具有足够的还原能力来介导CO 2 RR,因此在正确的配位环境中可以具有选择性。本报告描述了我们通过氧化还原活性配体修饰策略和氧化还原介体 (RM) 的使用来开发和优化新型 Cr 基 CO 2 RR 催化剂系统的努力。 RM 是氧化还原活性分子,可以在电化学反应过程中起辅助催化作用,将电子(通常伴随着质子)转移到催化活性位点。通过机械和计算工作,我们发现基于配体的氧化还原活性是控制这些 Cr 化合物对 CO 2活化的内在选择性的关键。基于配体的氧化还原活性对于开发共催化系统也至关重要,因为它能够与含有氧化还原活性平面芳族基团的还原性 RM 进行空间相互作用,从而允许在催化剂组件内发生电荷转移。在总结我们的工作之后,我们对未来开发用于小分子活化的早期过渡金属催化和助催化系统的可能性提出了展望。































 京公网安备 11010802027423号
京公网安备 11010802027423号