当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protocellular Heme and Iron–Sulfur Clusters
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-08-05 , DOI: 10.1021/acs.accounts.4c00254 Daniele Rossetto 1, 2 , Nemanja Cvjetan 1, 3 , Peter Walde 3 , Sheref S Mansy 1, 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-08-05 , DOI: 10.1021/acs.accounts.4c00254 Daniele Rossetto 1, 2 , Nemanja Cvjetan 1, 3 , Peter Walde 3 , Sheref S Mansy 1, 2
Affiliation
|
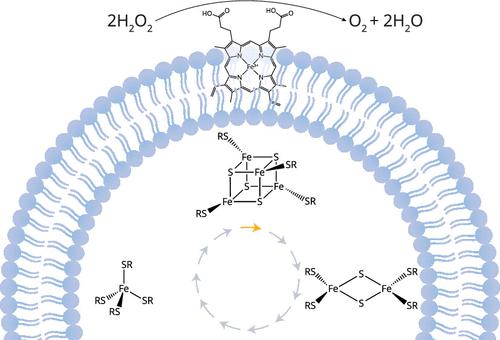
Central to the quest of understanding the emergence of life is to uncover the role of metals, particularly iron, in shaping prebiotic chemistry. Iron, as the most abundant of the accessible transition metals on the prebiotic Earth, played a pivotal role in early biochemical processes and continues to be indispensable to modern biology. Here, we discuss our recent contributions to probing the plausibility of prebiotic complexes with iron, including heme and iron–sulfur clusters, in mediating chemistry beneficial to a protocell. Laboratory experiments and spectroscopic findings suggest plausible pathways, often facilitated by UV light, for the synthesis of heme and iron–sulfur clusters. Once formed, heme displays catalytic, peroxidase-like activity when complexed with amphiphiles. This activity could have been beneficial in two ways. First, heme could have catalytically removed a molecule (H2O2) that could have had degradative effects on a protocell. Second, heme could have helped in the synthesis of the building blocks of life by coupling the reduction of H2O2 with the oxidation of organic substrates. The necessity of amphiphiles to avoid the formation of inactive complexes of heme is telling, as the modern-day electron transport chain possesses heme embedded within a lipid membrane. Conversely, prebiotic iron–sulfur peptides have yet to be reported to partition into lipid membranes, nor have simple iron–sulfur peptides been found to be capable of participating in the synthesis of organic molecules. Instead, iron–sulfur peptides span a wide range of reduction potentials complementary to the reduction potentials of hemes. The reduction potential of iron–sulfur peptides can be tuned by the type of iron–sulfur cluster formed, e.g., [2Fe-2S] versus [4Fe-4S], or by the substitution of ligands to the metal center. Since iron–sulfur clusters easily form upon stochastic encounters between iron ions, hydrosulfide, and small organic molecules possessing a thiolate, including peptides, the likelihood of soluble iron–sulfur clusters seems to be high. What remains challenging to determine is if iron–sulfur peptides participated in early prebiotic chemistry or were recruited later when protocellular membranes evolved that were compatible with the exploitation of electron transfer for the storage of energy as a proton gradient. This problem mirrors in some ways the difficulty in deciphering the origins of metabolism as a whole. Chemistry that resembles some facets of extant metabolism must have transpired on the prebiotic Earth, but there are few clues as to how and when such chemistry was harnessed to support a (proto)cell. Ultimately, unraveling the roles of hemes and iron–sulfur clusters in prebiotic chemistry promises to deepen our understanding of the origins of life on Earth and aids the search for life elsewhere in the universe.
中文翻译:

原生细胞血红素和铁硫簇
探索生命出现的核心是揭示金属,尤其是铁,在塑造益生元化学中的作用。铁作为益生元地球上最丰富的过渡金属,在早期生化过程中发挥了关键作用,并继续成为现代生物学不可或缺的一部分。在这里,我们讨论了我们最近对探索益生元复合物与铁的合理性的贡献,包括血红素和铁硫簇,在介导对原生细胞有益的化学中。实验室实验和光谱学研究结果表明,合成血红素和铁硫簇的途径通常是由紫外光促成的。一旦形成,血红素在与两亲物复合时表现出催化性、过氧化物酶样活性。这项活动可能从两个方面受益。首先,血红素可能催化去除了一个可能对原生细胞产生降解作用的分子 (H2O2)。其次,血红素可能通过将 H2O2 的还原与有机底物的氧化耦合来帮助合成生命的组成部分。两亲物避免形成血红素的无活性复合物的必要性很能说明问题,因为现代电子传递链具有嵌入脂质膜内的血红素。相反,尚未报道益生元铁硫肽会分配到脂质膜中,也尚未发现简单的铁硫肽能够参与有机分子的合成。相反,铁-硫肽具有广泛的还原电位,与血红素的还原电位互补。铁硫肽的还原电位可以通过形成的铁硫簇的类型进行调整,例如、[2Fe-2S] 与 [4Fe-4S] 的比较,或者通过配体取代金属中心。由于铁离子、硫化氢和具有硫酸盐的小有机分子(包括肽)之间随机相遇时很容易形成铁-硫簇,因此可溶性铁-硫簇的可能性似乎很高。仍然难以确定的是,铁硫肽是参与了早期的益生元化学,还是在后来的原生细胞膜进化时被募集,这与利用电子转移作为质子梯度来储存能量相兼容。这个问题在某种程度上反映了破译整个新陈代谢起源的困难。类似于现存新陈代谢的某些方面的化学反应一定发生在益生元地球上,但关于这种化学反应如何以及何时被利用来支持(原始)细胞的线索很少。最终,揭示血红素和铁硫簇在益生元化学中的作用有望加深我们对地球生命起源的理解,并有助于在宇宙其他地方寻找生命。
更新日期:2024-08-05
中文翻译:

原生细胞血红素和铁硫簇
探索生命出现的核心是揭示金属,尤其是铁,在塑造益生元化学中的作用。铁作为益生元地球上最丰富的过渡金属,在早期生化过程中发挥了关键作用,并继续成为现代生物学不可或缺的一部分。在这里,我们讨论了我们最近对探索益生元复合物与铁的合理性的贡献,包括血红素和铁硫簇,在介导对原生细胞有益的化学中。实验室实验和光谱学研究结果表明,合成血红素和铁硫簇的途径通常是由紫外光促成的。一旦形成,血红素在与两亲物复合时表现出催化性、过氧化物酶样活性。这项活动可能从两个方面受益。首先,血红素可能催化去除了一个可能对原生细胞产生降解作用的分子 (H2O2)。其次,血红素可能通过将 H2O2 的还原与有机底物的氧化耦合来帮助合成生命的组成部分。两亲物避免形成血红素的无活性复合物的必要性很能说明问题,因为现代电子传递链具有嵌入脂质膜内的血红素。相反,尚未报道益生元铁硫肽会分配到脂质膜中,也尚未发现简单的铁硫肽能够参与有机分子的合成。相反,铁-硫肽具有广泛的还原电位,与血红素的还原电位互补。铁硫肽的还原电位可以通过形成的铁硫簇的类型进行调整,例如、[2Fe-2S] 与 [4Fe-4S] 的比较,或者通过配体取代金属中心。由于铁离子、硫化氢和具有硫酸盐的小有机分子(包括肽)之间随机相遇时很容易形成铁-硫簇,因此可溶性铁-硫簇的可能性似乎很高。仍然难以确定的是,铁硫肽是参与了早期的益生元化学,还是在后来的原生细胞膜进化时被募集,这与利用电子转移作为质子梯度来储存能量相兼容。这个问题在某种程度上反映了破译整个新陈代谢起源的困难。类似于现存新陈代谢的某些方面的化学反应一定发生在益生元地球上,但关于这种化学反应如何以及何时被利用来支持(原始)细胞的线索很少。最终,揭示血红素和铁硫簇在益生元化学中的作用有望加深我们对地球生命起源的理解,并有助于在宇宙其他地方寻找生命。































 京公网安备 11010802027423号
京公网安备 11010802027423号