当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Borozenes: Benzene-Like Planar Aromatic Boron Clusters
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-08-03 , DOI: 10.1021/acs.accounts.4c00380 Lai-Sheng Wang 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-08-03 , DOI: 10.1021/acs.accounts.4c00380 Lai-Sheng Wang 1
Affiliation
|
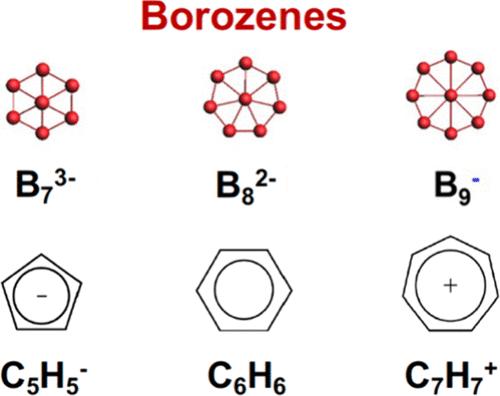
With three valence electrons and four valence orbitals, boron (2s22p1) is an electron-deficient element, resulting in interesting chemical bonding and structures in both borane molecules and bulk boron materials. The electron deficiency leads to electron sharing and delocalization in borane compounds and bulk boron allotropes, characterized by polyhedral cages, in particular, the ubiquitous B12 icosahedral cage. During the past two decades, the structures and bonding of size-selected boron clusters have been elucidated via combined photoelectron spectroscopy and theoretical investigations. Unlike bulk boron materials, finite boron clusters have been found to possess 2D structures consisting of B3 triangles, dotted with tetragonal, pentagonal, or hexagonal holes. The discovery of the planar B36 cluster with a central hexagonal hole provided the first experimental evidence for the viability of 2D boron nanostructures (borophene), which have been synthesized on inert substrates. The B7–, B8–, and B9– clusters were among the first few boron clusters to be investigated by joint photoelectron spectroscopy and theoretical calculations, and they were all found to possess 2D structures with a central B atom inside a Bn ring. Recently, the B73– (C6v), B82– (D7h), and B9– (D8h) series of closed-shell species were shown to possess similar π bonding akin to that in the C5H5–, C6H6, and C7H7+ series, respectively, and the name “borozene” was coined to highlight their analogy to the classical aromatic hydrocarbon molecules.
中文翻译:

硼烯:类苯平面芳香硼簇
硼 (2s 2 2p 1 ) 具有三个价电子和四个价轨道,是一种缺电子元素,可在硼烷分子和块状硼材料中产生有趣的化学键和结构。电子缺乏导致硼烷化合物和块体硼同素异形体中的电子共享和离域,其特征是多面体笼,特别是普遍存在的B 12二十面体笼。在过去的二十年中,通过结合光电子能谱和理论研究,已经阐明了尺寸选定的硼团簇的结构和键合。与块状硼材料不同,有限硼团簇被发现具有由B 3三角形组成的二维结构,其中散布着四边形、五边形或六边形孔。具有中心六角形孔的平面 B 36团簇的发现为二维硼纳米结构(硼烯)的可行性提供了第一个实验证据,该结构是在惰性基底上合成的。 B 7 – 、B 8 –和B 9 –团簇是最早通过联合光电子能谱和理论计算研究的几个硼团簇之一,并且它们都被发现具有二维结构,其中B n内有一个中心B原子。戒指。 最近,B 7 3– ( C 6 v )、B 8 2– ( D 7 h ) 和 B 9 – ( D 8 h ) 系列闭壳物种被证明具有与分别为C 5 H 5 – 、C 6 H 6和C 7 H 7 +系列,“硼氮烯”这个名称是为了突出它们与经典芳香烃分子的相似性而创造的。
更新日期:2024-08-03
中文翻译:

硼烯:类苯平面芳香硼簇
硼 (2s 2 2p 1 ) 具有三个价电子和四个价轨道,是一种缺电子元素,可在硼烷分子和块状硼材料中产生有趣的化学键和结构。电子缺乏导致硼烷化合物和块体硼同素异形体中的电子共享和离域,其特征是多面体笼,特别是普遍存在的B 12二十面体笼。在过去的二十年中,通过结合光电子能谱和理论研究,已经阐明了尺寸选定的硼团簇的结构和键合。与块状硼材料不同,有限硼团簇被发现具有由B 3三角形组成的二维结构,其中散布着四边形、五边形或六边形孔。具有中心六角形孔的平面 B 36团簇的发现为二维硼纳米结构(硼烯)的可行性提供了第一个实验证据,该结构是在惰性基底上合成的。 B 7 – 、B 8 –和B 9 –团簇是最早通过联合光电子能谱和理论计算研究的几个硼团簇之一,并且它们都被发现具有二维结构,其中B n内有一个中心B原子。戒指。 最近,B 7 3– ( C 6 v )、B 8 2– ( D 7 h ) 和 B 9 – ( D 8 h ) 系列闭壳物种被证明具有与分别为C 5 H 5 – 、C 6 H 6和C 7 H 7 +系列,“硼氮烯”这个名称是为了突出它们与经典芳香烃分子的相似性而创造的。































 京公网安备 11010802027423号
京公网安备 11010802027423号