Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Referenced Au Nanoparticles-Coated Glass Wafers for In Situ SERS Monitoring of Cell Secretion
ACS Sensors ( IF 8.2 ) Pub Date : 2024-08-05 , DOI: 10.1021/acssensors.4c01092 Zedong Zhang 1 , Chang Liu 1 , Jianguo Dong 1 , Aonan Zhu 2 , Chunyan An 1 , Dekun Wang 1 , Xue Mi 1 , Shijiing Yue 1 , Xiaoyue Tan 1 , Yuying Zhang 1
ACS Sensors ( IF 8.2 ) Pub Date : 2024-08-05 , DOI: 10.1021/acssensors.4c01092 Zedong Zhang 1 , Chang Liu 1 , Jianguo Dong 1 , Aonan Zhu 2 , Chunyan An 1 , Dekun Wang 1 , Xue Mi 1 , Shijiing Yue 1 , Xiaoyue Tan 1 , Yuying Zhang 1
Affiliation
|
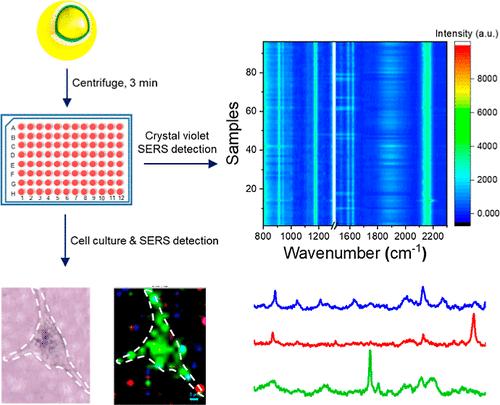
Surface-enhanced Raman spectroscopy (SERS) is a powerful technique for discrimination of bimolecules in complex systems. However, its practical applications face challenges such as complicated manufacturing procedures and limited scalability of SERS substrates, as well as poor reproducibility during detection which compromises the reliability of SERS-based analysis. In this study, we developed a convenient method for simultaneous fabrication of massive SERS substrates with an internal standard to eliminate the substrate-to-substrate differences. We first synthesized Au@CN@Au nanoparticles (NPs) which contain embedded internal standard molecules with a single characteristic peak in the Raman-silent region, and then deposited the NPs on 6 mm glass wafers in a 96-well plate simply by centrifugation for 3 min. The one-time obtained 96 SERS substrates have excellent intrasubstrate uniformity and intersubstrate repeatability for SERS detection by using the internal standard (relative standard deviation = 10.47%), and were able to detect both charged and neutral molecules (crystal violet and triphenylphosphine) at a concentration of 10–9 M. Importantly, cells can be directly cultured on glass wafers in the 96-well plate, enabling real time monitoring of the secretes and metabolism change in response to external stimulation. We found that the release of nucleic acids, amino acids and lipids by MDA-MB-231 cells significantly increased under hypoxic conditions. Overall, our approach enables fast and large-scale production of Au@CN@Au NPs-coated glass wafers as SERS substrates, which are homogeneous and highly sensitive for monitoring trace changes of biomolecules.
中文翻译:

用于原位 SERS 监测细胞分泌的自参金纳米颗粒涂层玻璃晶片
表面增强拉曼光谱 (SERS) 是一种用于区分复杂系统中双分子的强大技术。然而,其实际应用面临着一些挑战,例如复杂的制造过程和SERS基底的可扩展性有限,以及检测过程中的再现性差,从而损害了基于SERS的分析的可靠性。在这项研究中,我们开发了一种方便的方法,可以同时制造带有内标的大规模 SERS 基底,以消除基底之间的差异。我们首先合成了 Au@CN@Au 纳米颗粒(NP),其中包含嵌入的内标分子,在拉曼沉默区域具有单个特征峰,然后通过简单的离心将纳米颗粒沉积在 96 孔板中的 6 mm 玻璃晶片上。 3分钟一次性获得的96个SERS底物具有良好的底内均匀性和底物间重复性,使用内标进行SERS检测(相对标准偏差=10.47%),并且能够在100℃下同时检测带电分子和中性分子(结晶紫和三苯膦)。浓度为 10 –9 M。重要的是,细胞可以直接在 96 孔板的玻璃片上培养,从而能够实时监测分泌物和代谢变化以响应外部刺激。我们发现MDA-MB-231细胞在缺氧条件下释放的核酸、氨基酸和脂质显着增加。总体而言,我们的方法能够快速大规模生产 Au@CN@Au NPs 涂层玻璃晶圆作为 SERS 基板,这些玻璃晶圆是均匀且高度敏感的,可用于监测生物分子的痕量变化。
更新日期:2024-08-05
中文翻译:

用于原位 SERS 监测细胞分泌的自参金纳米颗粒涂层玻璃晶片
表面增强拉曼光谱 (SERS) 是一种用于区分复杂系统中双分子的强大技术。然而,其实际应用面临着一些挑战,例如复杂的制造过程和SERS基底的可扩展性有限,以及检测过程中的再现性差,从而损害了基于SERS的分析的可靠性。在这项研究中,我们开发了一种方便的方法,可以同时制造带有内标的大规模 SERS 基底,以消除基底之间的差异。我们首先合成了 Au@CN@Au 纳米颗粒(NP),其中包含嵌入的内标分子,在拉曼沉默区域具有单个特征峰,然后通过简单的离心将纳米颗粒沉积在 96 孔板中的 6 mm 玻璃晶片上。 3分钟一次性获得的96个SERS底物具有良好的底内均匀性和底物间重复性,使用内标进行SERS检测(相对标准偏差=10.47%),并且能够在100℃下同时检测带电分子和中性分子(结晶紫和三苯膦)。浓度为 10 –9 M。重要的是,细胞可以直接在 96 孔板的玻璃片上培养,从而能够实时监测分泌物和代谢变化以响应外部刺激。我们发现MDA-MB-231细胞在缺氧条件下释放的核酸、氨基酸和脂质显着增加。总体而言,我们的方法能够快速大规模生产 Au@CN@Au NPs 涂层玻璃晶圆作为 SERS 基板,这些玻璃晶圆是均匀且高度敏感的,可用于监测生物分子的痕量变化。































 京公网安备 11010802027423号
京公网安备 11010802027423号