Synlett ( IF 1.7 ) Pub Date : 2024-08-05 , DOI: 10.1055/a-2360-6586 Balaji Dashrath Sathe , Meenakshi Meenakshi 1 , Yogesh Murti 2 , Madhav Shivaji Mane 3 , Sarvesh Kumar Pandey 4 , Shriya Mahajan 5 , Pramod Rawat , Harsimrat Kandhari 6 , Kapil Kumar Goel 7 , ashish ranjan dwivedi 8 , S. V. Rathod 9
|
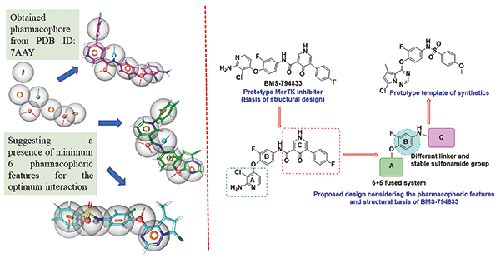
Mer proto-oncogene tyrosine-protein kinase (MerTK), a part of the TAM (TYRO3, AXL, and MerTK) family, is directly correlated with metastasis and various types of cancers. The inhibition of this receptor is a promising strategy for more-effective chemotherapy. Considering the pharmacophoric features of the active domain of MerTK and the structural characteristics of the investigational drug BMS794833, we designed five new N-{4-[(7-chloro-5-methylpyrrolo[2,1-f][1,2,4]triazin-4-yl)oxy]-3-fluorophenyl}benzenesulfonamide analogues. In cytotoxicity studies, one of the analogues displayed a significantly higher cytotoxicity than cisplatin. It showed IC50 values of 2.09, 1.96, and 3.08 μM against A549, MCF-7, and MDA-MB-231 cancer cell lines, respectively. In drug metabolism and pharmacokinetic studies, it was the most stable analogue and displayed a moderate MerTK inhibitory potential. Molecular-docking studies were performed to corroborate the MerTK inhibition, and the same analogue achieved the most significant docking score (–12.33 kcal/mol). Docking interactions demonstrated that the imine and amine group of the 3-chloropyridine moiety of BMS794833 formed hydrogen bonds with the main chain of the ATP pocket residue Met674, while the oxygen atoms of the 4-oxo-1,4-dihydropyridine-3-carboxamide moiety established hydrogen bonds with the Lys619 and Asp741 amino acid residues of the allosteric pocket of MerTK protein. These promising results provide evidence that the N-{4-[(7-chloro-5-methylpyrrolo[2,1-f][1,2,4]triazin-4-yl)oxy]-3-fluorophenyl}benzenesulfonamide pharmacophore can give potential insights into the development of new MerTK inhibitors.
中文翻译:

新型 N-{4-[(7-氯-5-甲基吡咯并[2,1-f] [1,2,4]三嗪-4-基)氧基]-3-氟苯基}苯磺酰胺类似物的开发:探索抗癌潜力通过 MerTK 抑制
Mer 原癌基因酪氨酸蛋白激酶 (MerTK) 是 TAM(TYRO3、AXL 和 MerTK)家族的一部分,与转移和各种类型的癌症直接相关。抑制该受体是一种有希望的更有效化疗策略。考虑到MerTK活性结构域的药效团特征以及在研药物BMS794833的结构特征,我们设计了5个新的N -{4-[(7-氯-5-甲基吡咯并[2,1- f ][1,2, 4]三嗪-4-基)氧基]-3-氟苯基}苯磺酰胺类似物。在细胞毒性研究中,其中一种类似物显示出比顺铂显着更高的细胞毒性。它对 A549、MCF-7 和 MDA-MB-231 癌细胞系的 IC 50值分别为 2.09、1.96 和 3.08 μM。在药物代谢和药代动力学研究中,它是最稳定的类似物,并表现出中等的 MerTK 抑制潜力。进行分子对接研究以证实 MerTK 抑制作用,相同的类似物获得了最显着的对接分数 (–12.33 kcal/mol)。对接相互作用表明,BMS794833的3-氯吡啶部分的亚胺和氨基与ATP袋残基Met674的主链形成氢键,而4-氧代-1,4-二氢吡啶-3-甲酰胺的氧原子形成氢键。该部分与 MerTK 蛋白变构袋的 Lys619 和 Asp741 氨基酸残基建立氢键。这些有希望的结果证明N -{4-[(7-氯-5-甲基吡咯并[2,1- f ][1,2,4]三嗪-4-基)氧基]-3-氟苯基}苯磺酰胺药效团可以为新型 MerTK 抑制剂的开发提供潜在的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号