当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A surface chemistry-regulated gradient multi-component solid electrolyte interphase for a 460 W h kg−1 lithium metal pouch cell
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-08-06 , DOI: 10.1039/d4ee02311k Man Pang 1 , Zhongwei Jiang 1 , Chongyang Luo 1 , Ziqing Yao 1 , Tianji Fu 1 , Tao Pan 1 , Qingpeng Guo 1 , Yujie Li 1 , Shizhao Xiong 2 , Chunman Zheng 1 , Weiwei Sun 1 , Guangmin Zhou 3 , Shuangke Liu 1
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-08-06 , DOI: 10.1039/d4ee02311k Man Pang 1 , Zhongwei Jiang 1 , Chongyang Luo 1 , Ziqing Yao 1 , Tianji Fu 1 , Tao Pan 1 , Qingpeng Guo 1 , Yujie Li 1 , Shizhao Xiong 2 , Chunman Zheng 1 , Weiwei Sun 1 , Guangmin Zhou 3 , Shuangke Liu 1
Affiliation
|
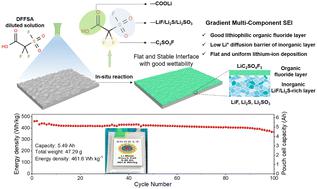
Lithium (Li) metal is an ideal anode for high energy density rechargeable Li batteries. However, parasitic reactions and an uneven native oxide layer on the surface lead to uncontrollable Li deposition and dendrite growth, significantly restricting its practical application. Here, we introduce a simple and scalable surface chemical approach involving spray casting of dilute 2,2-difluoro-2-(fluorosulfonyl)acetic acid (DFFSA) solution onto the Li surface, meticulously regulating ion transfer and improving interface stability to achieve stable cycling of the Li anode. The spontaneous in situ reaction between Li and DFFSA eliminates the uneven native oxide layer, forming an organic fluorinated carboxylate lithium salt on the outermost surface and a graded inorganic layer composed of LiF, Li2S, and Li2SO3 inside, resulting in a multi-component artificial solid electrolyte interphase (SEI). This multi-component SEI, as evidenced by visualization techniques and computational methods, exhibits enhanced Li affinity and wettability, enabling rapid lithium-ion transport and dendrite-free, uniform lithium deposition. Consequently, the modified Li||LiCoO2 full cell retains 77.85% capacity after 1200 cycles in carbonate-based electrolytes. A 461.6 W h kg−1 pouch cell with a capacity of 5.49 A h, a low N/P ratio of 1.28 and a lean electrolyte of 1.6 g A h−1, demonstrates an impressive capacity retention of 84.7% after 100 cycles at 0.5C. This work provides a simple and promising surface engineering strategy and enlightens the multi-component SEI design for promoting the practical application of high energy density Li metal batteries.
中文翻译:

用于 460 W h kg−1 锂金属软包电池的表面化学调节梯度多组分固体电解质界面
锂(Li)金属是高能量密度可充电锂电池的理想阳极。然而,寄生反应和表面不均匀的原生氧化层导致不可控的锂沉积和枝晶生长,严重限制了其实际应用。在这里,我们介绍了一种简单且可扩展的表面化学方法,包括将稀释的2,2-二氟-2-(氟磺酰基)乙酸(DFFSA)溶液喷射到Li表面上,精心调节离子转移并提高界面稳定性以实现稳定的循环Li阳极。 Li和DFFSA之间自发的原位反应消除了不均匀的原生氧化层,在最外表面形成有机氟化羧酸盐锂盐,在内部形成由LiF、Li 2 S和Li 2 SO 3组成的梯度无机层,从而形成多组分人造固体电解质界面(SEI)。可视化技术和计算方法证明,这种多组分 SEI 具有增强的锂亲和力和润湿性,可实现快速锂离子传输和无枝晶、均匀的锂沉积。因此,改进的Li||LiCoO 2全电池在碳酸酯基电解质中循环1200次后仍保持77.85%的容量。容量为 5.49 A h、低 N/P 比为 1.28 且贫电解液为 1.6 g A h -1的 461.6 W h kg -1软包电池在 0.5 次循环 100 次后显示出令人印象深刻的容量保持率为 84.7%。 C. 这项工作提供了一种简单且有前景的表面工程策略,并为促进高能量密度锂金属电池的实际应用的多组分SEI设计提供了启发。
更新日期:2024-08-06
中文翻译:

用于 460 W h kg−1 锂金属软包电池的表面化学调节梯度多组分固体电解质界面
锂(Li)金属是高能量密度可充电锂电池的理想阳极。然而,寄生反应和表面不均匀的原生氧化层导致不可控的锂沉积和枝晶生长,严重限制了其实际应用。在这里,我们介绍了一种简单且可扩展的表面化学方法,包括将稀释的2,2-二氟-2-(氟磺酰基)乙酸(DFFSA)溶液喷射到Li表面上,精心调节离子转移并提高界面稳定性以实现稳定的循环Li阳极。 Li和DFFSA之间自发的原位反应消除了不均匀的原生氧化层,在最外表面形成有机氟化羧酸盐锂盐,在内部形成由LiF、Li 2 S和Li 2 SO 3组成的梯度无机层,从而形成多组分人造固体电解质界面(SEI)。可视化技术和计算方法证明,这种多组分 SEI 具有增强的锂亲和力和润湿性,可实现快速锂离子传输和无枝晶、均匀的锂沉积。因此,改进的Li||LiCoO 2全电池在碳酸酯基电解质中循环1200次后仍保持77.85%的容量。容量为 5.49 A h、低 N/P 比为 1.28 且贫电解液为 1.6 g A h -1的 461.6 W h kg -1软包电池在 0.5 次循环 100 次后显示出令人印象深刻的容量保持率为 84.7%。 C. 这项工作提供了一种简单且有前景的表面工程策略,并为促进高能量密度锂金属电池的实际应用的多组分SEI设计提供了启发。































 京公网安备 11010802027423号
京公网安备 11010802027423号