Developmental Cell ( IF 10.7 ) Pub Date : 2024-08-05 , DOI: 10.1016/j.devcel.2024.07.008 Agnes Ulfig 1 , Ursula Jakob 2
|
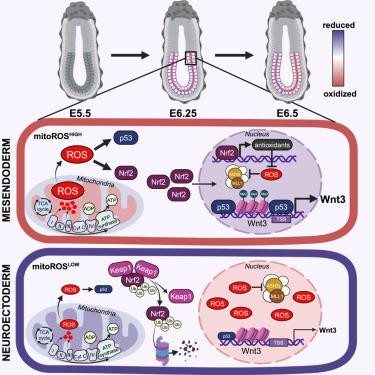
Pluripotent embryonic stem cells (ESCs) can develop into any cell type in the body. Yet, the regulatory mechanisms that govern cell fate decisions during embryogenesis remain largely unknown. We now demonstrate that mouse ESCs (mESCs) display large natural variations in mitochondrial reactive oxygen species (mitoROS) levels that individualize their nuclear redox state, H3K4me3 landscape, and cell fate. While mESCs with high mitoROS levels (mitoROSHIGH) differentiate toward mesendoderm and form the primitive streak during gastrulation, mESCs, which generate less ROS, choose the alternative neuroectodermal fate. Temporal studies demonstrated that mesendodermal (ME) specification of mitoROSHIGH mESCs is mediated by a Nrf2-controlled switch in the nuclear redox state, triggered by the accumulation of redox-sensitive H3K4me3 marks, and executed by a hitherto unknown ROS-dependent activation process of the Wnt signaling pathway. In summary, our study explains how ESC heterogeneity is generated and used by individual cells to decide between distinct cellular fates.
中文翻译:

小鼠胚胎干细胞的氧化还原异质性使细胞命运决定个体化
多能胚胎干细胞(ESC)可以发育成体内的任何细胞类型。然而,胚胎发生过程中控制细胞命运决定的调节机制仍然很大程度上未知。我们现在证明,小鼠 ESC (mESC) 在线粒体活性氧 (mitoROS) 水平方面表现出巨大的自然变化,从而使它们的核氧化还原状态、H3K4me3 景观和细胞命运个体化。虽然具有高 mitoROS 水平 (mitoROS HIGH ) 的 mESC 向中内胚层分化并在原肠胚形成期间形成原条,但产生较少 ROS 的 mESC 选择了替代的神经外胚层命运。时间研究表明,mitoROS HIGH mESC 的中内胚层 (ME) 规范是由 Nrf2 控制的核氧化还原状态开关介导的,由氧化还原敏感的 H3K4me3 标记的积累触发,并由迄今为止未知的 ROS 依赖性激活过程执行。 Wnt 信号通路。总之,我们的研究解释了 ESC 异质性是如何产生的,并被单个细胞用来决定不同的细胞命运。































 京公网安备 11010802027423号
京公网安备 11010802027423号