Chem Catalysis ( IF 11.5 ) Pub Date : 2024-08-05 , DOI: 10.1016/j.checat.2024.101069 Phebe H. van Langevelde , Dennis G.H. Hetterscheid
|
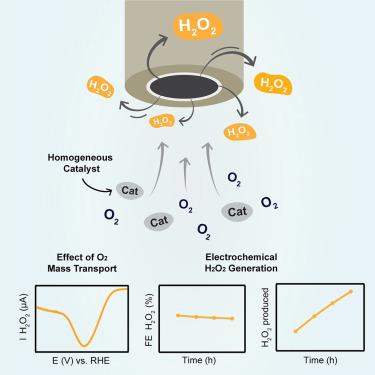
Hydrogen peroxide (H2O2) generation via electrochemical oxygen reduction is a sustainable production method for this bulk chemical. However, the selectivity of molecular catalysts for electrochemical H2O2 generation has hardly been investigated in a systematic manner, and it is unknown if their stability is sufficient for H2O2 generation in bulk electrolysis. This study answers these questions using the copper-based Cu(tmpa) (tmpa = tris(2-pyridylmethyl)amine) complex. Since the selectivity of H2O2 production originates from the relative rates of oxygen and H2O2 reduction, we show that substrate availability and catalyst concentration are key descriptors to tune the selectivity. Consequently, we can control the Faradaic efficiency to H2O2 (FEH2O2) in bulk electrolysis, and micromolar concentrations of Cu(tmpa) are sufficient for H2O2 production with an FEH2O2 of more than 50% over 8 h. Additionally, we show that Cu(tmpa) has a great electrochemical stability and is able to generate H2O2 in various electrolytes.
中文翻译:

分子铜催化剂选择性电化学 H2O2 生产:反应速率和传质之间的关键关系
通过电化学氧还原产生过氧化氢 (H 2 O 2 ) 是这种大宗化学品的可持续生产方法。然而,分子催化剂对电化学H 2 O 2生成的选择性几乎没有系统地研究,并且尚不清楚它们的稳定性是否足以在本体电解中生成H 2 O 2 。本研究使用铜基 Cu(tmpa)(tmpa = 三(2-吡啶基甲基)胺)络合物回答了这些问题。由于H 2 O 2生产的选择性源自氧气和H 2 O 2还原的相对速率,我们表明底物可用性和催化剂浓度是调节选择性的关键描述符。因此,我们可以控制本体电解中H 2 O 2 (FE H2O2 )的法拉第效率,微摩尔浓度的Cu(tmpa)足以在8小时内生产H 2 O 2 ,FE H2O2超过50%。此外,我们还发现Cu(tmpa)具有良好的电化学稳定性,能够在各种电解质中产生H 2 O 2 。































 京公网安备 11010802027423号
京公网安备 11010802027423号