当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
p-TsOH-Mediated Intramolecular C2-Arylation on NH-Indoles: Access of 5,10-Dihydroindeno[1,2-b]indoles
Organic Letters ( IF 4.9 ) Pub Date : 2024-08-05 , DOI: 10.1021/acs.orglett.4c02051 Anurag Verma 1, 2 , Ruchir Kant 3 , Nayan Ghosh 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2024-08-05 , DOI: 10.1021/acs.orglett.4c02051 Anurag Verma 1, 2 , Ruchir Kant 3 , Nayan Ghosh 1, 2
Affiliation
|
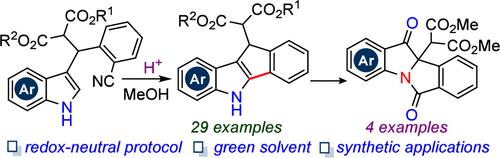
5,10-Dihydroindeno[1,2-b]indole has served as an important starting precursor for BARAC-fluor reagent in medicinal chemistry. Herein, an unprecedented p-TsOH assisted intramolecular C2-arylation of NH-indoles via C(sp2)-CN/C(sp2)-H coupling, offering a series of 5,10-dihydroindeno[1,2-b]indoles with moderate to good yields, has been showcased under redox-neutral conditions. Furthermore, successful scalability and synthetic applications highlight the practical nature of the method.
中文翻译:

p-TsOH 介导的 NH-吲哚分子内 C2-芳基化:获得 5,10-二氢茚并[1,2-b]吲哚
5,10-二氢茚并[1,2- b ]吲哚已成为药物化学中BARAC-氟试剂的重要起始前体。在此,前所未有的p -TsOH通过C(sp 2 )-CN/C(sp 2 )-H偶联辅助NH-吲哚的分子内C2-芳基化,提供了一系列5,10-二氢茚并[1,2- b ]在氧化还原中性条件下,吲哚具有中等至良好的产率。此外,成功的可扩展性和合成应用凸显了该方法的实用性。
更新日期:2024-08-05
中文翻译:

p-TsOH 介导的 NH-吲哚分子内 C2-芳基化:获得 5,10-二氢茚并[1,2-b]吲哚
5,10-二氢茚并[1,2- b ]吲哚已成为药物化学中BARAC-氟试剂的重要起始前体。在此,前所未有的p -TsOH通过C(sp 2 )-CN/C(sp 2 )-H偶联辅助NH-吲哚的分子内C2-芳基化,提供了一系列5,10-二氢茚并[1,2- b ]在氧化还原中性条件下,吲哚具有中等至良好的产率。此外,成功的可扩展性和合成应用凸显了该方法的实用性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号