当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Inhibition of Human Thymidylate Kinase and Structural Insights into the Phosphate Binding Loop and Ligand-Induced Degradation
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2016-10-28 00:00:00 , DOI: 10.1021/acs.jmedchem.6b01280 Yi-Hsuan Chen,Hua-Yi Hsu,Ming-Tyng Yeh,Chen-Cheng Chen,Chang-Yu Huang,Ying-Hsuan Chung,Zee-Fen Chang,Wei-Chen Kuo,Nei-Li Chan,Jui-Hsia Weng,Bon-chu Chung,Yu-Ju Chen,Cheng-Bang Jian,Ching-Chieh Shen,Hwan-Ching Tai,Sheh-Yi Sheu,Jim-Min Fang
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2016-10-28 00:00:00 , DOI: 10.1021/acs.jmedchem.6b01280 Yi-Hsuan Chen,Hua-Yi Hsu,Ming-Tyng Yeh,Chen-Cheng Chen,Chang-Yu Huang,Ying-Hsuan Chung,Zee-Fen Chang,Wei-Chen Kuo,Nei-Li Chan,Jui-Hsia Weng,Bon-chu Chung,Yu-Ju Chen,Cheng-Bang Jian,Ching-Chieh Shen,Hwan-Ching Tai,Sheh-Yi Sheu,Jim-Min Fang
|
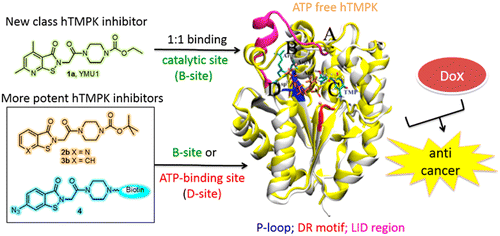
Targeting thymidylate kinase (TMPK) that catalyzes the phosphotransfer reaction for formation of dTDP from dTMP is a new strategy for anticancer treatment. This study is to understand the inhibitory mechanism of a previously identified human TMPK (hTMPK) inhibitor YMU1 (1a) by molecular docking, isothermal titration calorimetry, and photoaffinity labeling. The molecular dynamics simulation suggests that 1a prefers binding at the catalytic site of hTMPK, whereas the hTMPK inhibitors that bear pyridino[d]isothiazolone or benzo[d]isothiazolone core structure in lieu of the dimethylpyridine-fused isothiazolone moiety in 1a can have access to both the ATP-binding and catalytic sites. The binding sites of hTMPK inhibitors were validated by photoaffinity labeling and mass spectrometric studies. Taking together, 1a and its analogues stabilize the conformation of ligand-induced degradation (LID) region of hTMPK and block the catalytic site or ATP-binding site, thus attenuating the ATP binding-induced closed conformation that is required for phosphorylation of dTMP.
中文翻译:

人胸苷酸激酶的化学抑制和对磷酸盐结合环和配体诱导降解的结构见解。
靶向催化磷酸转移反应以从dTMP形成dTDP的胸苷酸激酶(TMPK)是抗癌治疗的新策略。这项研究旨在通过分子对接,等温滴定量热法和光亲和标记来了解先前鉴定的人TMPK(hTMPK)抑制剂YMU1(1a)的抑制机制。分子动力学模拟表明1a倾向于在hTMPK的催化位点结合,而带有吡啶并[ d ]异噻唑酮或苯并[ d ]异噻唑酮核心结构的hTMPK抑制剂代替1a中的二甲基吡啶稠合的异噻唑酮部分可以同时访问ATP结合位点和催化位点。hTMPK抑制剂的结合位点通过光亲和标记和质谱研究进行了验证。总而言之,1a及其类似物可稳定hTMPK的配体诱导的降解(LID)区的构象,并阻断催化位点或ATP结合位点,从而减弱dTMP磷酸化所需的ATP结合诱导的闭合构象。
更新日期:2016-10-28
中文翻译:

人胸苷酸激酶的化学抑制和对磷酸盐结合环和配体诱导降解的结构见解。
靶向催化磷酸转移反应以从dTMP形成dTDP的胸苷酸激酶(TMPK)是抗癌治疗的新策略。这项研究旨在通过分子对接,等温滴定量热法和光亲和标记来了解先前鉴定的人TMPK(hTMPK)抑制剂YMU1(1a)的抑制机制。分子动力学模拟表明1a倾向于在hTMPK的催化位点结合,而带有吡啶并[ d ]异噻唑酮或苯并[ d ]异噻唑酮核心结构的hTMPK抑制剂代替1a中的二甲基吡啶稠合的异噻唑酮部分可以同时访问ATP结合位点和催化位点。hTMPK抑制剂的结合位点通过光亲和标记和质谱研究进行了验证。总而言之,1a及其类似物可稳定hTMPK的配体诱导的降解(LID)区的构象,并阻断催化位点或ATP结合位点,从而减弱dTMP磷酸化所需的ATP结合诱导的闭合构象。































 京公网安备 11010802027423号
京公网安备 11010802027423号