当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Homogenous Electrocatalytic Oxygen Reduction Rates Correlate with Reaction Overpotential in Acidic Organic Solutions
ACS Central Science ( IF 12.7 ) Pub Date : 2016-10-28 00:00:00 , DOI: 10.1021/acscentsci.6b00261 Michael L. Pegis 1 , Bradley A. McKeown 1 , Neeraj Kumar 2 , Kai Lang 3 , Derek J. Wasylenko 4 , X. Peter Zhang 5 , Simone Raugei 2 , James M. Mayer 1
ACS Central Science ( IF 12.7 ) Pub Date : 2016-10-28 00:00:00 , DOI: 10.1021/acscentsci.6b00261 Michael L. Pegis 1 , Bradley A. McKeown 1 , Neeraj Kumar 2 , Kai Lang 3 , Derek J. Wasylenko 4 , X. Peter Zhang 5 , Simone Raugei 2 , James M. Mayer 1
Affiliation
|
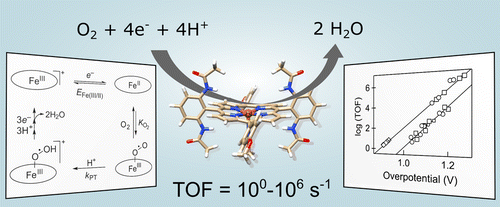
Improved electrocatalysts for the oxygen reduction reaction (ORR) are critical for the advancement of fuel cell technologies. Herein, we report a series of 11 soluble iron porphyrin ORR electrocatalysts that possess turnover frequencies (TOFs) from 3 s–1 to an unprecedented value of 2.2 × 106 s–1. These TOFs correlate with the ORR overpotential, which can be modulated by changing the E1/2 of the catalyst using different ancillary ligands, by changing the solvent and solution acidity, and by changing the catalyst’s protonation state. The overpotential is well-defined for these homogeneous electrocatalysts by the E1/2 of the catalyst and the proton activity of the solution. This is the first such correlation for homogeneous ORR electrocatalysis, and it demonstrates that the remarkably fast TOFs are a consequence of high overpotential. The correlation with overpotential is surprising since the turnover limiting steps involve oxygen binding and protonation, as opposed to turnover limiting electron transfer commonly found in Tafel analysis of heterogeneous ORR materials. Computational studies show that the free energies for oxygen binding to the catalyst and for protonation of the superoxide complex are in general linearly related to the catalyst E1/2, and that this is the origin of the overpotential correlations. This analysis thus provides detailed understanding of the ORR barriers. The best catalysts involve partial decoupling of the influence of the second coordination sphere from the properties of the metal center, which is suggested as new molecular design strategy to avoid the limitations of the traditional scaling relationships for these catalysts.
中文翻译:

酸性有机溶液中均相电催化氧还原速率与反应超电势相关
用于氧还原反应(ORR)的改进的电催化剂对于燃料电池技术的发展至关重要。在此,我们报告了一系列11种可溶性铁卟啉ORR电催化剂,它们的转换频率(TOF)从3 s –1到前所未有的2.2×10 6 s –1。这些TOF与ORR过电势相关,可以通过使用不同的辅助配体来改变催化剂的E 1/2,通过改变溶剂和溶液的酸度以及通过改变催化剂的质子化状态来调节。这些均相电催化剂的超电势由E 1/2定义得很清楚催化剂的性质和溶液的质子活性。这是均相ORR电催化的第一个这样的相关性,它证明了极快的TOF是高过电势的结果。与超电势的相关性令人惊讶,因为周转限制步骤涉及氧键合和质子化,这与异质ORR材料的Tafel分析中通常发现的周转限制电子转移相反。计算研究表明,氧与催化剂结合和超氧化物配合物的质子化的自由能通常与催化剂E 1/2线性相关。,这就是超电位相关性的起源。因此,该分析提供了对ORR障碍的详细理解。最好的催化剂涉及第二配位球的影响与金属中心性能的部分解耦,这被建议作为一种新的分子设计策略,以避免这些催化剂的传统比例关系的局限性。
更新日期:2016-10-28
中文翻译:

酸性有机溶液中均相电催化氧还原速率与反应超电势相关
用于氧还原反应(ORR)的改进的电催化剂对于燃料电池技术的发展至关重要。在此,我们报告了一系列11种可溶性铁卟啉ORR电催化剂,它们的转换频率(TOF)从3 s –1到前所未有的2.2×10 6 s –1。这些TOF与ORR过电势相关,可以通过使用不同的辅助配体来改变催化剂的E 1/2,通过改变溶剂和溶液的酸度以及通过改变催化剂的质子化状态来调节。这些均相电催化剂的超电势由E 1/2定义得很清楚催化剂的性质和溶液的质子活性。这是均相ORR电催化的第一个这样的相关性,它证明了极快的TOF是高过电势的结果。与超电势的相关性令人惊讶,因为周转限制步骤涉及氧键合和质子化,这与异质ORR材料的Tafel分析中通常发现的周转限制电子转移相反。计算研究表明,氧与催化剂结合和超氧化物配合物的质子化的自由能通常与催化剂E 1/2线性相关。,这就是超电位相关性的起源。因此,该分析提供了对ORR障碍的详细理解。最好的催化剂涉及第二配位球的影响与金属中心性能的部分解耦,这被建议作为一种新的分子设计策略,以避免这些催化剂的传统比例关系的局限性。































 京公网安备 11010802027423号
京公网安备 11010802027423号