Developmental Cell ( IF 10.7 ) Pub Date : 2024-08-01 , DOI: 10.1016/j.devcel.2024.07.004 Chiara De Leonibus 1 , Marianna Maddaluno 2 , Rosa Ferriero 3 , Roberta Besio 4 , Laura Cinque 2 , Pei Jin Lim 5 , Alessandro Palma 3 , Rossella De Cegli 3 , Salvatore Gagliotta 3 , Sandro Montefusco 3 , Maria Iavazzo 2 , Marianne Rohrbach 5 , Cecilia Giunta 5 , Elena Polishchuk 3 , Diego Louis Medina 6 , Diego Di Bernardo 7 , Antonella Forlino 4 , Pasquale Piccolo 3 , Carmine Settembre 2
|
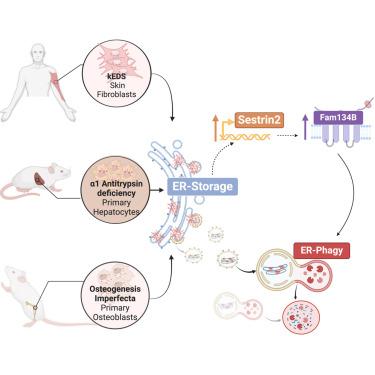
Protein biogenesis within the endoplasmic reticulum (ER) is crucial for organismal function. Errors during protein folding necessitate the removal of faulty products. ER-associated protein degradation and ER-phagy target misfolded proteins for proteasomal and lysosomal degradation. The mechanisms initiating ER-phagy in response to ER proteostasis defects are not well understood. By studying mouse primary cells and patient samples as a model of ER storage disorders (ERSDs), we show that accumulation of faulty products within the ER triggers a response involving SESTRIN2, a nutrient sensor controlling mTORC1 signaling. SESTRIN2 induction by XBP1 inhibits mTORC1’s phosphorylation of TFEB/TFE3, allowing these transcription factors to enter the nucleus and upregulate the ER-phagy receptor FAM134B along with lysosomal genes. This response promotes ER-phagy of misfolded proteins via FAM134B-Calnexin complex. Pharmacological induction of FAM134B improves clearance of misfolded proteins in ERSDs. Our study identifies the interplay between nutrient signaling and ER quality control, suggesting therapeutic strategies for ERSDs.
中文翻译:

Sestrin2 响应蛋白质错误折叠驱动 ER 自噬
内质网 (ER) 内的蛋白质生物发生对机体功能至关重要。蛋白质折叠过程中的错误需要去除有缺陷的产品。ER 相关蛋白降解和 ER 自噬靶向错误折叠的蛋白质进行蛋白酶体和溶酶体降解。响应 ER 蛋白稳态缺陷而启动 ER 自噬的机制尚不清楚。通过研究小鼠原代细胞和患者样本作为 ER 贮积症 (ERSD) 的模型,我们表明 ER 内缺陷产物的积累会触发涉及 SESTRIN2 的反应, 是一种控制 mTORC1 信号传导的营养传感器。XBP1 的SESTRIN2诱导抑制 mTORC1 对 TFEB/TFE3 的磷酸化,使这些转录因子进入细胞核并上调 ER 自噬受体FAM134B与溶酶体基因。这种反应通过 FAM134B-钙联结蛋白复合物促进错误折叠蛋白的 ER 自噬。FAM134B 的药理学诱导可改善 ERSDS 中错误折叠蛋白的清除。我们的研究确定了营养信号传导和 ER 质量控制之间的相互作用,提出了 ERSD 的治疗策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号