当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, Antifungal Activity, and Mechanism of Action of New Piperidine-4-carbohydrazide Derivatives Bearing a Quinazolinyl Moiety
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-07-29 , DOI: 10.1021/acs.jafc.4c03860 Yehui Yang 1 , Songsong Liu 1 , Taisen Yan 1 , Mingyan Yi 1 , Hong Li 1 , Xiaoping Bao 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-07-29 , DOI: 10.1021/acs.jafc.4c03860 Yehui Yang 1 , Songsong Liu 1 , Taisen Yan 1 , Mingyan Yi 1 , Hong Li 1 , Xiaoping Bao 1
Affiliation
|
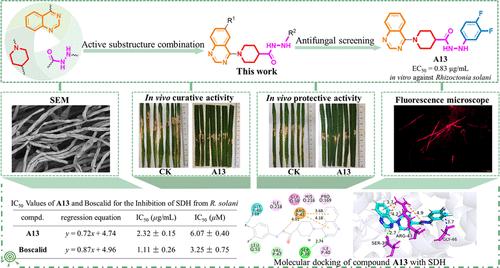
A series of new piperidine-4-carbohydrazide derivatives bearing a quinazolinyl moiety were prepared and evaluated for their fungicidal activities against agriculturally important fungi. Among these derivatives, the chemical structure of compound A45 was clearly verified by X-ray crystallographic analysis. The antifungal bioassays revealed that many compounds in this series possessed good to excellent inhibition effects toward the tested fungi. For example, compounds A13 and A41 had EC50 values of 0.83 and 0.88 μg/mL against Rhizoctonia solani in vitro, respectively, superior to those of positive controls Chlorothalonil and Boscalid (1.64 and 0.96 μg/mL, respectively). Additionally, the above two compounds also exhibited notable inhibitory activities against Verticillium dahliae (with EC50 values of 1.12 and 3.20 μg/mL, respectively), far better than the positive controls Carbendazim and Chlorothalonil (19.3 and 11.0 μg/mL, respectively). More importantly, compound A13 could potently inhibit the proliferation of R. solani in the potted rice plants, showing good in vivo curative and protective efficiencies of 76.9% and 76.6% at 200 μg/mL, respectively. Furthermore, compound A13 demonstrated an effective inhibition of succinate dehydrogenase (SDH) activity in vitro with an IC50 value of 6.07 μM. Finally, the molecular docking study revealed that this compound could be well embedded into the active pocket of SDH via multiple noncovalent interactions, involving residues like SER39, ARG43, and GLY46.
中文翻译:

带有喹唑啉基的新型哌啶-4-碳酰肼衍生物的设计、合成、抗真菌活性和作用机制
制备了一系列带有喹唑啉基部分的新型哌啶-4-碳酰肼衍生物,并评估了它们对农业重要真菌的杀真菌活性。在这些衍生物中,化合物A45的化学结构通过X射线晶体分析得到了清晰的验证。抗真菌生物测定表明,该系列中的许多化合物对受试真菌具有良好至优异的抑制作用。例如,化合物A13和A41对立枯丝核菌的体外EC 50值分别为0.83和0.88μg/mL,优于阳性对照百菌清和啶酰菌胺(分别为1.64和0.96μg/mL)。此外,上述两种化合物还对大丽黄萎病表现出显着的抑制活性(EC 50值分别为1.12和3.20 μg/mL),远优于阳性对照多菌灵和百菌清(分别为19.3和11.0 μg/mL)。更重要的是,化合物A13可以有效抑制盆栽水稻中立枯丝核菌的增殖,在200 μg/mL时表现出良好的体内治疗和保护效率,分别为76.9%和76.6%。此外,化合物A13在体外有效抑制琥珀酸脱氢酶(SDH)活性,IC 50值为6.07 μM。最后,分子对接研究表明,该化合物可以通过涉及 SER39、ARG43 和 GLY46 等残基的多种非共价相互作用很好地嵌入 SDH 的活性口袋中。
更新日期:2024-07-29
中文翻译:

带有喹唑啉基的新型哌啶-4-碳酰肼衍生物的设计、合成、抗真菌活性和作用机制
制备了一系列带有喹唑啉基部分的新型哌啶-4-碳酰肼衍生物,并评估了它们对农业重要真菌的杀真菌活性。在这些衍生物中,化合物A45的化学结构通过X射线晶体分析得到了清晰的验证。抗真菌生物测定表明,该系列中的许多化合物对受试真菌具有良好至优异的抑制作用。例如,化合物A13和A41对立枯丝核菌的体外EC 50值分别为0.83和0.88μg/mL,优于阳性对照百菌清和啶酰菌胺(分别为1.64和0.96μg/mL)。此外,上述两种化合物还对大丽黄萎病表现出显着的抑制活性(EC 50值分别为1.12和3.20 μg/mL),远优于阳性对照多菌灵和百菌清(分别为19.3和11.0 μg/mL)。更重要的是,化合物A13可以有效抑制盆栽水稻中立枯丝核菌的增殖,在200 μg/mL时表现出良好的体内治疗和保护效率,分别为76.9%和76.6%。此外,化合物A13在体外有效抑制琥珀酸脱氢酶(SDH)活性,IC 50值为6.07 μM。最后,分子对接研究表明,该化合物可以通过涉及 SER39、ARG43 和 GLY46 等残基的多种非共价相互作用很好地嵌入 SDH 的活性口袋中。






























 京公网安备 11010802027423号
京公网安备 11010802027423号