Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical and DFT insights into 2-amino-4-(4-hydroxy-3-methoxyphenyl)-7-methyl-4H-chromene-3-carbonitrile: an innovative strategy for antibacterial activity and corrosion protection of carbon steel
RSC Advances ( IF 3.9 ) Pub Date : 2024-08-02 , DOI: 10.1039/d4ra03785e Badreah A Al Jahdaly 1
RSC Advances ( IF 3.9 ) Pub Date : 2024-08-02 , DOI: 10.1039/d4ra03785e Badreah A Al Jahdaly 1
Affiliation
|
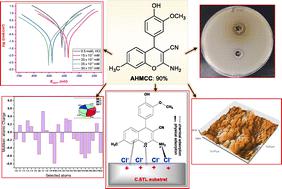
This study explored the potential of a newly synthesized derivative, 2-amino-4-(4-hydroxy-3-methoxyphenyl)-7-methyl-4H-chromene-3-carbonitrile (AHMCC), as a broad-spectrum antibacterial agent and a corrosion inhibitor for carbon steel (C.STL) in 0.5 M HCl solution. AHMCC demonstrated remarkable antibacterial efficacy against Gram-negative (Escherichia coli, Klebsiella pneumoniae) and Gram-positive (Bacillus subtilis, Staphylococcus aureus) bacteria, as evidenced by agar plate tests and cell viability assays. In the corrosion inhibition studies, AHMCC exhibited mixed-type inhibitor behavior as revealed by potentiodynamic polarization (PDP) measurements. The inhibition efficiency increased with rising AHMCC concentration, confirmed by a significant enhancement in charge transfer resistance (Rct) observed in electrochemical impedance spectroscopy (EIS) analysis. Electrochemical frequency modulation (EFM) data with obtained CF2 and CF3 values further corroborated these findings. Langmuir isotherm modeling suggested AHMCC molecules followed a monolayer adsorption pattern on the C.STL surface. UV-visible spectroscopy indicated the formation of a protective layer through chemical interaction between AHMCC and the metal surface. Atomic force microscopy (AFM) provided visual confirmation of this protective film shielding the C.STL from the corrosive environment. Additionally, theoretical calculations supported the proposed adsorption mechanism of AHMCC molecules onto the C.STL surface.
中文翻译:

对 2-氨基-4-(4-羟基-3-甲氧基苯基)-7-甲基-4H-色烯-3-甲腈的电化学和 DFT 见解:碳钢抗菌活性和腐蚀保护的创新策略
本研究探讨了新合成的衍生物 2-氨基-4-(4-羟基-3-甲氧基苯基)-7-甲基-4 H-色烯-3-甲腈 ( AHMCC ) 作为广谱抗菌剂的潜力以及 0.5 M HCl 溶液中的碳钢缓蚀剂 (C.STL)。琼脂平板试验和细胞活力测定证明, AHMCC对革兰氏阴性菌(大肠杆菌、肺炎克雷伯菌)和革兰氏阳性菌(枯草芽孢杆菌、金黄色葡萄球菌)具有显着的抗菌功效。在腐蚀抑制研究中,动电位极化 (PDP) 测量表明, AHMCC表现出混合型缓蚀剂行为。抑制效率随着AHMCC浓度的增加而增加,电化学阻抗谱 (EIS) 分析中观察到的电荷转移电阻 ( R ct ) 显着增强证实了这一点。获得的CF 2和CF 3值的电化学频率调制(EFM) 数据进一步证实了这些发现。 Langmuir 等温线模型表明AHMCC分子在 C.STL 表面上遵循单层吸附模式。紫外-可见光谱表明AHMCC和金属表面之间通过化学相互作用形成了保护层。原子力显微镜 (AFM) 提供了该保护膜的视觉确认,该保护膜保护 C.STL 免受腐蚀环境的影响。 此外,理论计算支持了所提出的AHMCC分子在 C.STL 表面上的吸附机制。
更新日期:2024-08-02
中文翻译:

对 2-氨基-4-(4-羟基-3-甲氧基苯基)-7-甲基-4H-色烯-3-甲腈的电化学和 DFT 见解:碳钢抗菌活性和腐蚀保护的创新策略
本研究探讨了新合成的衍生物 2-氨基-4-(4-羟基-3-甲氧基苯基)-7-甲基-4 H-色烯-3-甲腈 ( AHMCC ) 作为广谱抗菌剂的潜力以及 0.5 M HCl 溶液中的碳钢缓蚀剂 (C.STL)。琼脂平板试验和细胞活力测定证明, AHMCC对革兰氏阴性菌(大肠杆菌、肺炎克雷伯菌)和革兰氏阳性菌(枯草芽孢杆菌、金黄色葡萄球菌)具有显着的抗菌功效。在腐蚀抑制研究中,动电位极化 (PDP) 测量表明, AHMCC表现出混合型缓蚀剂行为。抑制效率随着AHMCC浓度的增加而增加,电化学阻抗谱 (EIS) 分析中观察到的电荷转移电阻 ( R ct ) 显着增强证实了这一点。获得的CF 2和CF 3值的电化学频率调制(EFM) 数据进一步证实了这些发现。 Langmuir 等温线模型表明AHMCC分子在 C.STL 表面上遵循单层吸附模式。紫外-可见光谱表明AHMCC和金属表面之间通过化学相互作用形成了保护层。原子力显微镜 (AFM) 提供了该保护膜的视觉确认,该保护膜保护 C.STL 免受腐蚀环境的影响。 此外,理论计算支持了所提出的AHMCC分子在 C.STL 表面上的吸附机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号