当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Safe and Practical Synthesis of Enantiomerically Pure (S)- and (R)-N-Boc-3-(Trifluoromethyl)piperazines Enabled by Aza-Michael Addition of Optically Pure 4-Phenyl-2-Oxazolidinone to 3,3,3-Trifluoro-1-Nitropropene
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-07-30 , DOI: 10.1021/acs.oprd.4c00289 Chetan Padmakar Darne 1 , Ning Li 1 , Daniel Smith 2 , Shasha Zhang 3 , Premsai Rai Neithnadka 4 , Arundutt Silamkoti 4 , Arunachalam Arumugam 4 , Anuradha Gupta 4 , Zhenqiu Hong 2 , Subramaniam Krishnananthan 2 , Bei Wang 2 , Rulin Zhao 2 , Shiuhang Yip 2 , Dauh-Rurng Wu 2 , James Kempson 2 , Deborah S. Mortensen 5 , Arvind Mathur 2 , Jianqing Li 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-07-30 , DOI: 10.1021/acs.oprd.4c00289 Chetan Padmakar Darne 1 , Ning Li 1 , Daniel Smith 2 , Shasha Zhang 3 , Premsai Rai Neithnadka 4 , Arundutt Silamkoti 4 , Arunachalam Arumugam 4 , Anuradha Gupta 4 , Zhenqiu Hong 2 , Subramaniam Krishnananthan 2 , Bei Wang 2 , Rulin Zhao 2 , Shiuhang Yip 2 , Dauh-Rurng Wu 2 , James Kempson 2 , Deborah S. Mortensen 5 , Arvind Mathur 2 , Jianqing Li 1
Affiliation
|
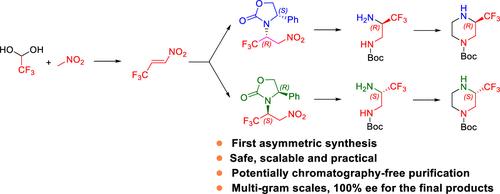
(S)- and (R)-N-Boc-3-(trifluoromethyl)piperazines are attractive building blocks for the rational design of drugs. Until now, their chiral syntheses were unknown. Exploration of three synthetic approaches via chiral 3,3,3-trifluoropropane-1,2-diamines yielded a scalable route that has been used for multigram synthesis. Two routes were not amendable for scale-up due to low yielding, tedious purification, limited availability of reagents, and safety issues. The successful and practical route relied on a modified process to mitigate the safety issues for the synthesis of (E)-3,3,3-trifluoro-1-nitroprop-1-ene, which was used as a stock solution for the highly diastereoselective aza-Michael addition of optically pure 4-phenyl-2-oxazolidinone. Boc protection of an amino group allowed subsequent transformations to chiral N-Boc-protected 3,3,3-trifluoropropane-1,2-diamine under mild conditions, without the need for chiral chromatography. The amidation of chiral N-Boc-protected 3,3,3-trifluoropropane-1,2-diamine with 2-chloroacetyl chloride, followed by intramolecular cyclization and subsequent reduction afforded enantiomerically pure N-Boc-3-(trifluoromethyl)piperazines.
中文翻译:

通过光学纯 4-苯基-2-恶唑烷酮与 3,3 的 Aza-Michael 加成实现对映体纯 (S)- 和 (R)-N-Boc-3-(三氟甲基)哌嗪的安全实用合成, 3-三氟-1-硝基丙烯
( S )-和( R ) -N -Boc-3-(三氟甲基)哌嗪是药物合理设计的有吸引力的构建模块。到目前为止,他们的手性合成尚不清楚。通过手性 3,3,3-三氟丙烷-1,2-二胺探索三种合成方法,产生了一条可扩展的路线,已用于多克合成。由于产量低、纯化繁琐、试剂可用性有限以及安全问题,两条路线无法修改以扩大规模。该成功且实用的路线依赖于改进的工艺来减轻合成( E )-3,3,3-三氟-1-硝基丙-1-烯的安全问题,该路线被用作高度非对映选择性的储备溶液光学纯4-苯基-2-恶唑烷酮的氮杂-迈克尔加成。氨基的 Boc 保护允许随后在温和条件下转化为手性N -Boc 保护的 3,3,3-三氟丙烷-1,2-二胺,无需手性色谱。手性N -Boc-保护的3,3,3-三氟丙烷-1,2-二胺与2-氯乙酰氯酰胺化,然后进行分子内环化和随后的还原,得到对映体纯的N -Boc-3-(三氟甲基)哌嗪。
更新日期:2024-07-30
中文翻译:

通过光学纯 4-苯基-2-恶唑烷酮与 3,3 的 Aza-Michael 加成实现对映体纯 (S)- 和 (R)-N-Boc-3-(三氟甲基)哌嗪的安全实用合成, 3-三氟-1-硝基丙烯
( S )-和( R ) -N -Boc-3-(三氟甲基)哌嗪是药物合理设计的有吸引力的构建模块。到目前为止,他们的手性合成尚不清楚。通过手性 3,3,3-三氟丙烷-1,2-二胺探索三种合成方法,产生了一条可扩展的路线,已用于多克合成。由于产量低、纯化繁琐、试剂可用性有限以及安全问题,两条路线无法修改以扩大规模。该成功且实用的路线依赖于改进的工艺来减轻合成( E )-3,3,3-三氟-1-硝基丙-1-烯的安全问题,该路线被用作高度非对映选择性的储备溶液光学纯4-苯基-2-恶唑烷酮的氮杂-迈克尔加成。氨基的 Boc 保护允许随后在温和条件下转化为手性N -Boc 保护的 3,3,3-三氟丙烷-1,2-二胺,无需手性色谱。手性N -Boc-保护的3,3,3-三氟丙烷-1,2-二胺与2-氯乙酰氯酰胺化,然后进行分子内环化和随后的还原,得到对映体纯的N -Boc-3-(三氟甲基)哌嗪。































 京公网安备 11010802027423号
京公网安备 11010802027423号