当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fully Continuous Flow Synthesis of 2′-Deoxy-2′-fluoro-arabinoside: A Key Intermediate of Azvudine
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-07-31 , DOI: 10.1021/acs.oprd.4c00166 Yan Chen 1 , Yongcheng Sun 1 , Yufang Xu 1 , Xuhong Qian 1 , Weiping Zhu 1, 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-07-31 , DOI: 10.1021/acs.oprd.4c00166 Yan Chen 1 , Yongcheng Sun 1 , Yufang Xu 1 , Xuhong Qian 1 , Weiping Zhu 1, 2
Affiliation
|
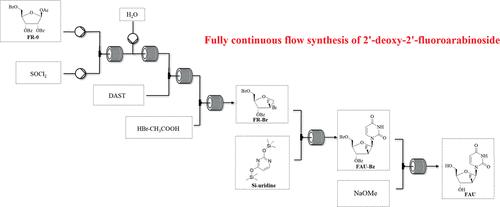
Azvudine was approved for the treatment of adult HIV-1 infection in China in 2021, and it was approved for conditional marketing for the treatment of SARS-CoV-2 in China in 2022. In this work, we describe a fully continuous flow synthesis of 2′-deoxy-2′-fluoroarabinoside, a key intermediate for azvudine. The process was accomplished via six chemical transformations, including chlorination, hydrolysis, fluorination, bromination, condensation, and deprotection in six sequential continuous flow devices. Under the optimized process conditions, the total yield was 32.3% with a total residence time of 156 min. Compared with batch conditions, the total yield was doubled, the total reaction time was shortened 16 times, and the E factor was reduced 1.63 times.
更新日期:2024-07-31






























 京公网安备 11010802027423号
京公网安备 11010802027423号