当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Lenacapavir Sodium: Active Pharmaceutical Ingredient Process Development and Scale-up
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-07-31 , DOI: 10.1021/acs.oprd.4c00242 Anna M. Wagner 1 , Sara A. Bonderoff 2 , Stanley Chang 2 , Jeffrey Deignan 1 , Michelle M. Esanu 2 , Huy Van Huynh 2 , Tianmin Niu 2 , Vinh Ngo 1 , B. Michael O’Keefe 1 , Jenny Phoenix 2 , Trevor J. Rainey 1 , Benjamin J. Roberts 1 , Jinyu Shen 2 , Craig Stewart 2 , Amanda L. Vandehey 1 , Scott A. Wolckenhauer 1 , Chloe Y. Wong 1 , Brian H. Yarmuch 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-07-31 , DOI: 10.1021/acs.oprd.4c00242 Anna M. Wagner 1 , Sara A. Bonderoff 2 , Stanley Chang 2 , Jeffrey Deignan 1 , Michelle M. Esanu 2 , Huy Van Huynh 2 , Tianmin Niu 2 , Vinh Ngo 1 , B. Michael O’Keefe 1 , Jenny Phoenix 2 , Trevor J. Rainey 1 , Benjamin J. Roberts 1 , Jinyu Shen 2 , Craig Stewart 2 , Amanda L. Vandehey 1 , Scott A. Wolckenhauer 1 , Chloe Y. Wong 1 , Brian H. Yarmuch 2
Affiliation
|
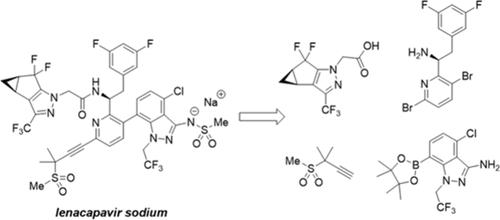
Lenacapavir sodium (GS-6207-02) is a first-in-class HIV capsid inhibitor. Process development of the four-step final assembly of lenacapavir sodium from four synthetic intermediates is described here. A bis-bromopyridine core is sequentially subjected to an alkynylation, an amide coupling with a chiral pyrazole carboxylic acid, and a Suzuki cross-coupling with an indazole boronic ester. The final step is a telescoped bis-methanesulfonylation and hydrolysis to yield the API. This report highlights experimental work on the final assembly sequence to establish robust processing conditions, minimize process mass intensity, control impurity formation, understand impurity purge, and enable large-scale manufacturing of lenacapavir sodium.
中文翻译:

来那卡韦钠的合成:活性药物成分工艺开发和放大
Lenacapvirodium (GS-6207-02) 是一种一流的 HIV 衣壳抑制剂。本文描述了由四种合成中间体四步最终组装来那卡韦钠的工艺开发。双溴吡啶核依次进行炔基化、与手性吡唑羧酸的酰胺偶联、以及与吲唑硼酸酯的铃木交叉偶联。最后一步是伸缩式双甲磺酰化和水解以产生 API。本报告重点介绍了最终组装序列的实验工作,以建立稳健的加工条件,最大限度地降低工艺质量强度,控制杂质形成,了解杂质清除,并实现来那卡韦钠的大规模生产。
更新日期:2024-07-31
中文翻译:

来那卡韦钠的合成:活性药物成分工艺开发和放大
Lenacapvirodium (GS-6207-02) 是一种一流的 HIV 衣壳抑制剂。本文描述了由四种合成中间体四步最终组装来那卡韦钠的工艺开发。双溴吡啶核依次进行炔基化、与手性吡唑羧酸的酰胺偶联、以及与吲唑硼酸酯的铃木交叉偶联。最后一步是伸缩式双甲磺酰化和水解以产生 API。本报告重点介绍了最终组装序列的实验工作,以建立稳健的加工条件,最大限度地降低工艺质量强度,控制杂质形成,了解杂质清除,并实现来那卡韦钠的大规模生产。






























 京公网安备 11010802027423号
京公网安备 11010802027423号