当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydroxyl on Stepped Copper and its Interaction with Water
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-07-30 , DOI: 10.1021/acs.jpcc.4c04091
Kallum Mistry 1 , Henry Snowden 1 , George R Darling 1 , Andrew Hodgson 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-07-30 , DOI: 10.1021/acs.jpcc.4c04091
Kallum Mistry 1 , Henry Snowden 1 , George R Darling 1 , Andrew Hodgson 1
Affiliation
|
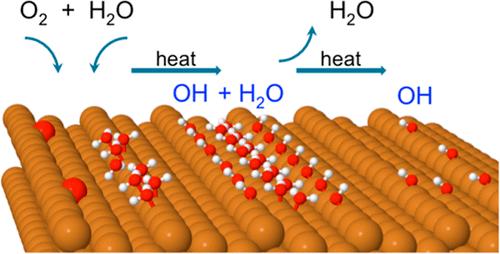
We describe the hydroxyl and mixed hydroxyl-water structures formed on a stepped copper surface following the reaction of adsorbed O with water at a low temperature and compare them to the structures found previously on plane copper surfaces. Thermal desorption profiles, STM, and low-energy electron diffraction show that water reacts with O at temperatures below 130 K on Cu(511). Two well-defined phases appear as the OH/H2O layer is heated to desorb excess water, a 1OH:1H2O phase and a pure OH phase. The 1OH:1H2O structure consists of 1D chains binding across two adjacent copper steps, with a double period along the step. Electronic structure calculations show that the structure has a zigzag chain of water along the terrace, stabilized by hydrogen bonds to OH groups adsorbed in the step bridge sites. This structure binds OH in its favored site and is similar to the structure observed on other open faces of Cu and Ni, suggesting that this structural arrangement may be common on other surfaces that have steps or rows of close packed metal atoms. The hydroxyl/water chains decompose at 210 K to leave OH adsorbed in the Cu step bridge site, with some forming H-bonded trimers that bridge between two Cu steps. Heating the surface causes hydroxyl to disproportionate near 300 K, desorbing water to leave chemisorbed O.
中文翻译:

阶梯铜上的羟基及其与水的相互作用
我们描述了吸附的 O 与水在低温下反应后在阶梯铜表面上形成的羟基和混合羟基-水结构,并将它们与之前在平面铜表面上发现的结构进行比较。热解吸曲线、STM 和低能电子衍射表明,水在低于 130 K 的温度下在 Cu(511) 上与 O 发生反应。当 OH/H 2 O 层被加热以解吸过量的水时,出现两个明确的相:1OH:1H 2 O 相和纯 OH 相。 1OH:1H 2 O 结构由跨两个相邻铜台阶结合的一维链组成,沿台阶具有双周期。电子结构计算表明,该结构沿着平台具有锯齿形的水链,通过与阶梯桥位点上吸附的羟基的氢键稳定。这种结构将 OH 结合在其有利的位置,并且与在 Cu 和 Ni 的其他开放面上观察到的结构相似,表明这种结构排列可能在具有阶梯或密排金属原子行的其他表面上很常见。羟基/水链在 210 K 下分解,留下吸附在 Cu 台阶桥位点上的 OH,其中一些形成氢键三聚体,在两个 Cu 台阶之间桥接。加热表面会导致羟基在 300 K 附近发生歧化,解吸水以留下化学吸附的 O。
更新日期:2024-07-30
中文翻译:

阶梯铜上的羟基及其与水的相互作用
我们描述了吸附的 O 与水在低温下反应后在阶梯铜表面上形成的羟基和混合羟基-水结构,并将它们与之前在平面铜表面上发现的结构进行比较。热解吸曲线、STM 和低能电子衍射表明,水在低于 130 K 的温度下在 Cu(511) 上与 O 发生反应。当 OH/H 2 O 层被加热以解吸过量的水时,出现两个明确的相:1OH:1H 2 O 相和纯 OH 相。 1OH:1H 2 O 结构由跨两个相邻铜台阶结合的一维链组成,沿台阶具有双周期。电子结构计算表明,该结构沿着平台具有锯齿形的水链,通过与阶梯桥位点上吸附的羟基的氢键稳定。这种结构将 OH 结合在其有利的位置,并且与在 Cu 和 Ni 的其他开放面上观察到的结构相似,表明这种结构排列可能在具有阶梯或密排金属原子行的其他表面上很常见。羟基/水链在 210 K 下分解,留下吸附在 Cu 台阶桥位点上的 OH,其中一些形成氢键三聚体,在两个 Cu 台阶之间桥接。加热表面会导致羟基在 300 K 附近发生歧化,解吸水以留下化学吸附的 O。








































 京公网安备 11010802027423号
京公网安备 11010802027423号