当前位置:
X-MOL 学术
›
Cell Stem Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acetyl-CoA metabolism maintains histone acetylation for syncytialization of human placental trophoblast stem cells
Cell Stem Cell ( IF 19.8 ) Pub Date : 2024-07-30 , DOI: 10.1016/j.stem.2024.07.003 Xin Yu 1 , Hao Wu 1 , Jiali Su 1 , Xupeng Liu 1 , Kun Liang 1 , Qianqian Li 1 , Ruoxuan Yu 1 , Xuan Shao 2 , Hongmei Wang 3 , Yan-Ling Wang 3 , Ng Shyh-Chang 3
Cell Stem Cell ( IF 19.8 ) Pub Date : 2024-07-30 , DOI: 10.1016/j.stem.2024.07.003 Xin Yu 1 , Hao Wu 1 , Jiali Su 1 , Xupeng Liu 1 , Kun Liang 1 , Qianqian Li 1 , Ruoxuan Yu 1 , Xuan Shao 2 , Hongmei Wang 3 , Yan-Ling Wang 3 , Ng Shyh-Chang 3
Affiliation
|
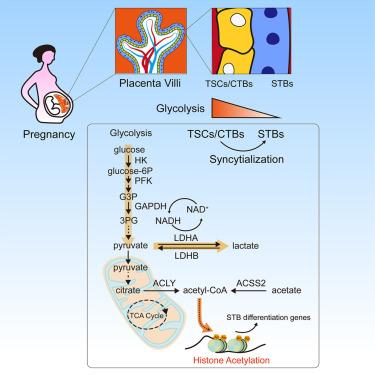
During pregnancy, placental-fetal nutrient allocation is crucial for fetal and maternal health. However, the regulatory mechanisms for nutrient metabolism and allocation in placental trophoblasts have remained unclear. Here, we used human first-trimester placenta samples and human trophoblast stem cells (hTSCs) to discover that glucose metabolism is highly active in hTSCs and cytotrophoblasts, but during syncytialization, it decreases to basal levels, remaining necessary for fueling acetyl-CoA and differentiation potential. Acetate supplementation could rescue syncytiotrophoblast fusion from glycolysis deficiency by replenishing acetyl-CoA and maintaining histone acetylation, thus rescuing the activation of syncytialization genes. Even brief glycolysis deficiency could permanently inhibit differentiation potential and promote inflammation, which could also be permanently rescued by brief acetate supplementation in vivo . These results suggest that hTSCs retain only basal glycolytic acetyl-CoA metabolism during syncytialization to regulate cell fates via nutrient-responsive histone acetylation, with implications for our understanding of the balance between placental and fetal nutrition.
中文翻译:

乙酰辅酶A代谢维持组蛋白乙酰化以促进人胎盘滋养层干细胞的合胞化
怀孕期间,胎盘-胎儿的营养分配对于胎儿和母亲的健康至关重要。然而,胎盘滋养层营养代谢和分配的调节机制仍不清楚。在这里,我们使用人类早孕期胎盘样本和人类滋养层干细胞 (hTSC) 发现,葡萄糖代谢在 hTSC 和细胞滋养层中高度活跃,但在合体化过程中,它降低至基础水平,仍然是促进乙酰辅酶 A 和分化所必需的潜在的。补充乙酸可以通过补充乙酰辅酶A和维持组蛋白乙酰化来挽救合体滋养层融合免受糖酵解缺陷的影响,从而挽救合体化基因的激活。即使是短暂的糖酵解缺陷也可能永久抑制分化潜力并促进炎症,这也可以通过体内短暂补充醋酸盐来永久挽救。这些结果表明,hTSC 在合胞化过程中仅保留基础糖酵解乙酰辅酶 A 代谢,通过营养响应性组蛋白乙酰化来调节细胞命运,这对我们了解胎盘和胎儿营养之间的平衡具有重要意义。
更新日期:2024-07-30
中文翻译:

乙酰辅酶A代谢维持组蛋白乙酰化以促进人胎盘滋养层干细胞的合胞化
怀孕期间,胎盘-胎儿的营养分配对于胎儿和母亲的健康至关重要。然而,胎盘滋养层营养代谢和分配的调节机制仍不清楚。在这里,我们使用人类早孕期胎盘样本和人类滋养层干细胞 (hTSC) 发现,葡萄糖代谢在 hTSC 和细胞滋养层中高度活跃,但在合体化过程中,它降低至基础水平,仍然是促进乙酰辅酶 A 和分化所必需的潜在的。补充乙酸可以通过补充乙酰辅酶A和维持组蛋白乙酰化来挽救合体滋养层融合免受糖酵解缺陷的影响,从而挽救合体化基因的激活。即使是短暂的糖酵解缺陷也可能永久抑制分化潜力并促进炎症,这也可以通过体内短暂补充醋酸盐来永久挽救。这些结果表明,hTSC 在合胞化过程中仅保留基础糖酵解乙酰辅酶 A 代谢,通过营养响应性组蛋白乙酰化来调节细胞命运,这对我们了解胎盘和胎儿营养之间的平衡具有重要意义。































 京公网安备 11010802027423号
京公网安备 11010802027423号