当前位置:
X-MOL 学术
›
Aliment. Pharm. Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficacy and safety of risankizumab by baseline corticosteroid use and achievement of corticosteroid‐free clinical and endoscopic outcomes in patients with moderately to severely active Crohn's disease
Alimentary Pharmacology & Therapeutics ( IF 6.6 ) Pub Date : 2024-07-26 , DOI: 10.1111/apt.18184 Stefan Schreiber 1 , Raymond K. Cross 2 , Remo Panaccione 3 , Geert D'Haens 4 , Peter Bossuyt 5 , Iris Dotan 6, 7 , Jean‐Frederic Colombel 8 , Edouard Louis 9 , Marla C. Dubinsky 8 , Kristina Kligys 10 , Ezequiel Neimark 10 , Alexandra Song 10 , Javier Zambrano 10 , Jasmina Kalabic 11 , Erica Cheng 10 , Yafei Zhang 10 , Marc Ferrante 12
Alimentary Pharmacology & Therapeutics ( IF 6.6 ) Pub Date : 2024-07-26 , DOI: 10.1111/apt.18184 Stefan Schreiber 1 , Raymond K. Cross 2 , Remo Panaccione 3 , Geert D'Haens 4 , Peter Bossuyt 5 , Iris Dotan 6, 7 , Jean‐Frederic Colombel 8 , Edouard Louis 9 , Marla C. Dubinsky 8 , Kristina Kligys 10 , Ezequiel Neimark 10 , Alexandra Song 10 , Javier Zambrano 10 , Jasmina Kalabic 11 , Erica Cheng 10 , Yafei Zhang 10 , Marc Ferrante 12
Affiliation
|
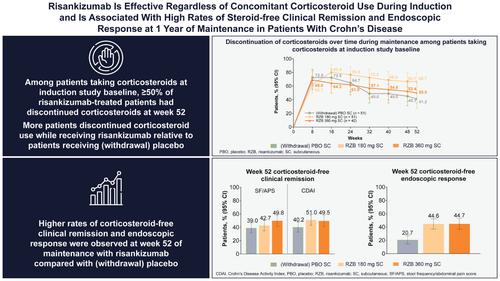
SummaryBackgroundRisankizumab is efficacious and well tolerated in adults with moderately to severely active Crohn's disease (CD).AimTo evaluate the corticosteroid‐sparing effect of risankizumab in CD.MethodsDuring the 12‐week induction period, patients maintained stable baseline corticosteroid doses, up to 20 mg/day prednisone or equivalent. At week 0 of maintenance, a mandatory corticosteroid taper was started. This post hoc analysis evaluated corticosteroid‐free clinical and endoscopic outcomes at week 52 of maintenance; safety was also assessed.ResultsOf 889 patients randomised to induction with risankizumab 600 mg or placebo, 285 (32.1%) were taking baseline concomitant corticosteroids. Week 12 clinical remission and endoscopic response rates were greater for risankizumab 600 mg versus placebo, regardless of concomitant corticosteroid use. At week 52, 66.7%, 50.0% and 41.2% of patients taking risankizumab 180 mg, risankizumab 360 mg and (withdrawal) placebo, respectively, discontinued corticosteroids. Week 52 corticosteroid‐free clinical remission per stool frequency/abdominal pain score (risankizumab 180 mg [42.7%] or 360 mg [49.8%]; [withdrawal] placebo [39.0%]), corticosteroid‐free clinical remission per Crohn's Disease Activity Index (risankizumab 180 mg [51.0%] or 360 mg [49.5%]; [withdrawal] placebo [40.2%]), and corticosteroid‐free endoscopic response (risankizumab 180 mg [44.6%] or 360 mg [44.7%]; [withdrawal] placebo [20.7%]) rates were greater for risankizumab than placebo. Adverse event rates were generally similar, regardless of baseline corticosteroid use.ConclusionsEfficacy of risankizumab 600 mg induction therapy was independent of concomitant corticosteroid use. Risankizumab 180 and 360 mg maintenance therapy yielded high rates of corticosteroid‐free clinical and endoscopic outcomes at week 52.
中文翻译:

中度至重度活动性克罗恩病患者基线皮质类固醇使用以及无皮质类固醇临床和内镜结果的 risankizumab 的疗效和安全性
摘要背景 Risankizumab 对患有中度至重度活动性克罗恩病 (CD) 的成人有效且耐受性良好。目的评估 risankizumab 在 CD 中的皮质类固醇节约效果。方法在 12 周诱导期内,患者维持稳定的基线皮质类固醇剂量,最高 20 mg /天泼尼松或等效物。在维持治疗的第 0 周,开始强制减少皮质类固醇剂量。这项事后分析评估了维持治疗第 52 周时无皮质类固醇的临床和内镜结果;还评估了安全性。 结果 在 889 名随机接受 risankizumab 600 mg 或安慰剂诱导的患者中,285 名 (32.1%) 正在服用基线伴随皮质类固醇。无论是否同时使用皮质类固醇,第 12 周 risankizumab 600 mg 的临床缓解率和内镜缓解率均高于安慰剂。在第 52 周,服用 risankizumab 180 mg、risankizumab 360 mg 和(停药)安慰剂的患者中分别有 66.7%、50.0% 和 41.2% 停用皮质类固醇。第 52 周,每次排便频率/腹痛评分无皮质类固醇临床缓解(risankizumab 180 mg [42.7%] 或 360 mg [49.8%];[停药]安慰剂 [39.0%]),每克罗恩病活动指数无皮质类固醇临床缓解(risankizumab 180 mg [51.0%] 或 360 mg [49.5%];[停药]安慰剂 [40.2%]),以及无皮质类固醇内窥镜反应(risankizumab 180 mg [44.6%] 或 360 mg [44.7%];[停药] ] 安慰剂 [20.7%])risankizumab 的发生率高于安慰剂。无论基线皮质类固醇的使用情况如何,不良事件发生率通常相似。 结论 risankizumab 600 mg 诱导治疗的疗效独立于伴随皮质类固醇的使用。 Risankizumab 180 和 360 mg 维持治疗在第 52 周产生了高比例的无皮质类固醇临床和内镜结果。
更新日期:2024-07-26
中文翻译:

中度至重度活动性克罗恩病患者基线皮质类固醇使用以及无皮质类固醇临床和内镜结果的 risankizumab 的疗效和安全性
摘要背景 Risankizumab 对患有中度至重度活动性克罗恩病 (CD) 的成人有效且耐受性良好。目的评估 risankizumab 在 CD 中的皮质类固醇节约效果。方法在 12 周诱导期内,患者维持稳定的基线皮质类固醇剂量,最高 20 mg /天泼尼松或等效物。在维持治疗的第 0 周,开始强制减少皮质类固醇剂量。这项事后分析评估了维持治疗第 52 周时无皮质类固醇的临床和内镜结果;还评估了安全性。 结果 在 889 名随机接受 risankizumab 600 mg 或安慰剂诱导的患者中,285 名 (32.1%) 正在服用基线伴随皮质类固醇。无论是否同时使用皮质类固醇,第 12 周 risankizumab 600 mg 的临床缓解率和内镜缓解率均高于安慰剂。在第 52 周,服用 risankizumab 180 mg、risankizumab 360 mg 和(停药)安慰剂的患者中分别有 66.7%、50.0% 和 41.2% 停用皮质类固醇。第 52 周,每次排便频率/腹痛评分无皮质类固醇临床缓解(risankizumab 180 mg [42.7%] 或 360 mg [49.8%];[停药]安慰剂 [39.0%]),每克罗恩病活动指数无皮质类固醇临床缓解(risankizumab 180 mg [51.0%] 或 360 mg [49.5%];[停药]安慰剂 [40.2%]),以及无皮质类固醇内窥镜反应(risankizumab 180 mg [44.6%] 或 360 mg [44.7%];[停药] ] 安慰剂 [20.7%])risankizumab 的发生率高于安慰剂。无论基线皮质类固醇的使用情况如何,不良事件发生率通常相似。 结论 risankizumab 600 mg 诱导治疗的疗效独立于伴随皮质类固醇的使用。 Risankizumab 180 和 360 mg 维持治疗在第 52 周产生了高比例的无皮质类固醇临床和内镜结果。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号