当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Integrated and Modular Compartmentalized Microfluidic System with Tunable Electrospun Porous Membranes for Epithelialized Organs-on-a-Chip
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-07-24 , DOI: 10.1021/acsami.4c08864 Roa S Fardous 1, 2 , Sultan Alshmmari 2, 3 , Essam Tawfik 4 , Ibrahim Khadra 1 , Qasem Ramadan 2 , Moahammed Zourob 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-07-24 , DOI: 10.1021/acsami.4c08864 Roa S Fardous 1, 2 , Sultan Alshmmari 2, 3 , Essam Tawfik 4 , Ibrahim Khadra 1 , Qasem Ramadan 2 , Moahammed Zourob 2
Affiliation
|
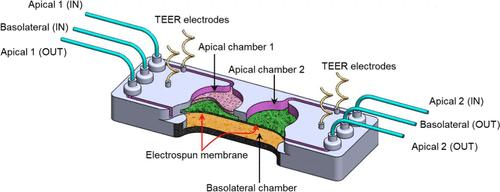
A modular and 3D compartmentalized microfluidic system with electrospun porous membranes (PMs) for epithelialized organ-on-a-chip systems is presented. Our novel approach involves direct deposition of polymer nanofibers onto a patterned poly(methyl methacrylate) (PMMA) substrate using electrospinning, resulting in an integrated PM within the microfluidic chip. The in situ deposition of the PM eliminates the need for additional assembly processes. To demonstrate the high throughput membrane integration capability of our approach, we successfully deposited nanofibers onto various chip designs with complex microfluidic planar structures and expanded dimensions. We characterized and tested the fully PMMA chip by growing an epithelial monolayer using the Caco-2 cell line to study drug permeability. A comprehensive analysis of the bulk and surface properties of the membrane’s fibers made of PMMA and polystyrene (PS) was conducted to determine the polymer with the best performance for cell culture and drug transport applications. The PMMA-based membrane, with a PMMA/PVP ratio of 5:1, allowed for the fabrication of a uniform membrane structure along the aligned nanofibers. By modulating the fiber diameter and total thickness of the membrane, we could adjust the membrane’s porosity for specific cell culture applications. The PMMA–PVP nanofibers exhibited a low polydispersity index value, indicating monodispersed nanofibers and a more homogeneous and uniform fiber network. Both types of membranes demonstrated excellent mechanical integrity under medium perfusion flow rates. However, the PMMA–PVP composition offered a tailored porous structure with modulable porosity based on the fiber diameter and thickness. Our developed platform enables dynamic in vitro modeling of the epithelial barrier and has applications in drug transport and in vitro microphysiological systems.
中文翻译:

具有可调谐电纺多孔膜的集成模块化隔室微流体系统,用于芯片上皮器官
提出了一种模块化和 3D 区室微流体系统,具有用于上皮器官芯片系统的电纺多孔膜 (PM)。我们的新颖方法涉及使用静电纺丝将聚合物纳米纤维直接沉积到图案化的聚甲基丙烯酸甲酯 (PMMA) 基板上,从而在微流控芯片内形成集成的 PM。 PM 的原位沉积消除了额外组装工艺的需要。为了证明我们的方法的高通量膜集成能力,我们成功地将纳米纤维沉积到具有复杂微流体平面结构和扩展尺寸的各种芯片设计上。我们通过使用 Caco-2 细胞系生长上皮单层来研究药物渗透性,对完整的 PMMA 芯片进行了表征和测试。对由 PMMA 和聚苯乙烯 (PS) 制成的膜纤维的体积和表面特性进行了全面分析,以确定最适合细胞培养和药物运输应用的聚合物。 PMMA 基膜的 PMMA/PVP 比例为 5:1,可以沿着排列的纳米纤维制造均匀的膜结构。通过调节膜的纤维直径和总厚度,我们可以调整膜的孔隙率以适应特定的细胞培养应用。 PMMA-PVP纳米纤维表现出较低的多分散指数值,表明纳米纤维是单分散的,并且纤维网络更加均匀一致。两种类型的膜在中等灌注流速下均表现出优异的机械完整性。然而,PMMA-PVP 组合物提供了定制的多孔结构,其孔隙率可根据纤维直径和厚度进行调节。 我们开发的平台能够对上皮屏障进行动态体外建模,并在药物转运和体外微生理系统中得到应用。
更新日期:2024-07-24
中文翻译:

具有可调谐电纺多孔膜的集成模块化隔室微流体系统,用于芯片上皮器官
提出了一种模块化和 3D 区室微流体系统,具有用于上皮器官芯片系统的电纺多孔膜 (PM)。我们的新颖方法涉及使用静电纺丝将聚合物纳米纤维直接沉积到图案化的聚甲基丙烯酸甲酯 (PMMA) 基板上,从而在微流控芯片内形成集成的 PM。 PM 的原位沉积消除了额外组装工艺的需要。为了证明我们的方法的高通量膜集成能力,我们成功地将纳米纤维沉积到具有复杂微流体平面结构和扩展尺寸的各种芯片设计上。我们通过使用 Caco-2 细胞系生长上皮单层来研究药物渗透性,对完整的 PMMA 芯片进行了表征和测试。对由 PMMA 和聚苯乙烯 (PS) 制成的膜纤维的体积和表面特性进行了全面分析,以确定最适合细胞培养和药物运输应用的聚合物。 PMMA 基膜的 PMMA/PVP 比例为 5:1,可以沿着排列的纳米纤维制造均匀的膜结构。通过调节膜的纤维直径和总厚度,我们可以调整膜的孔隙率以适应特定的细胞培养应用。 PMMA-PVP纳米纤维表现出较低的多分散指数值,表明纳米纤维是单分散的,并且纤维网络更加均匀一致。两种类型的膜在中等灌注流速下均表现出优异的机械完整性。然而,PMMA-PVP 组合物提供了定制的多孔结构,其孔隙率可根据纤维直径和厚度进行调节。 我们开发的平台能够对上皮屏障进行动态体外建模,并在药物转运和体外微生理系统中得到应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号