当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nucleophilic Aromatic Substitution of Halobenzenes and Phenols with Catalysis by Arenophilic π Acids
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-07-23 , DOI: 10.1021/acs.accounts.4c00327 Kai Chen 1 , Hang Shi 1, 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-07-23 , DOI: 10.1021/acs.accounts.4c00327 Kai Chen 1 , Hang Shi 1, 2
Affiliation
|
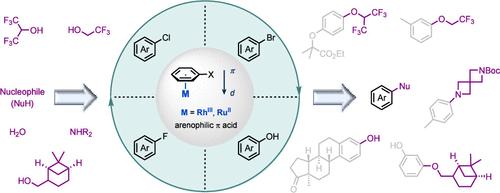
Lewis π acids, particularly high-valent transition metals with vacant orbitals, can coordinate with unsaturated compounds such as alkynes and alkenes by means of π-bonding. The coordination enhances the electrophilicity of the bound compounds, thereby facilitating reactions─such as nucleophilic addition─that take place at the ligated carbon–carbon multiple bonds. This activation phenomenon occurs at the ligand rather than at the metal atom, and it has been extensively utilized in the development of catalytic methods. In addition to alkynes and alkenes, aromatic compounds featuring a phenyl ring can be activated by an electrophilic transition-metal unit (e.g., Cr(CO)3, [Mn(CO)3]+, [CpFe]+, or [CpRu]+, where Cp = cyclopentadienyl) through π coordination. Over the past several decades, remarkable advances have been achieved in the development of reactions occurring on bound arenes, capitalizing on the highly electron-withdrawing nature of these transition-metal units and on the thermodynamic stability of η6-arene complexes. A prime example is the extension of nucleophilic aromatic substitution (SNAr) reactions to electron-neutral and -rich halobenzenes. Such arenes, which are normally inert to classical SNAr, can undergo sequences involving complex formation, substitution, and complex decomposition. Despite the successes achieved through the utilization of preformed complexes, the application of reversible arene coordination to catalytic systems has seen only limited progress. Consequently, in π-coordination activation, transition-metal units are commonly considered to be components of bound arene complexes rather than π-acid catalysts.
中文翻译:

亲核π酸催化卤代苯和苯酚的亲核芳香取代
路易斯π酸,特别是具有空轨道的高价过渡金属,可以通过π键与炔烃、烯烃等不饱和化合物配位。配位增强了结合化合物的亲电性,从而促进在连接的碳-碳多重键上发生的反应(例如亲核加成)。这种活化现象发生在配体而不是金属原子上,并且已广泛用于催化方法的开发。除了炔烃和烯烃之外,具有苯环的芳香族化合物也可以通过亲电过渡金属单元(例如 Cr(CO) 3 、[Mn(CO) 3 ] + 、[CpFe] +或 [CpRu] 活化) + ,其中 Cp = 环戊二烯基)通过 π 配位。在过去的几十年中,利用这些过渡金属单元的高吸电子性质和 η 6 -芳烃配合物的热力学稳定性,在结合芳烃上发生的反应的发展方面取得了显着的进展。一个典型的例子是将亲核芳族取代( SN Ar)反应扩展到电子中性和富卤代苯。此类芳烃通常对经典 S N Ar 呈惰性,可以经历涉及络合物形成、取代和络合物分解的序列。尽管通过利用预制配合物取得了成功,但可逆芳烃配位在催化系统中的应用仅取得有限的进展。因此,在π配位活化中,过渡金属单元通常被认为是结合芳烃配合物的组分,而不是π酸催化剂。
更新日期:2024-07-23
中文翻译:

亲核π酸催化卤代苯和苯酚的亲核芳香取代
路易斯π酸,特别是具有空轨道的高价过渡金属,可以通过π键与炔烃、烯烃等不饱和化合物配位。配位增强了结合化合物的亲电性,从而促进在连接的碳-碳多重键上发生的反应(例如亲核加成)。这种活化现象发生在配体而不是金属原子上,并且已广泛用于催化方法的开发。除了炔烃和烯烃之外,具有苯环的芳香族化合物也可以通过亲电过渡金属单元(例如 Cr(CO) 3 、[Mn(CO) 3 ] + 、[CpFe] +或 [CpRu] 活化) + ,其中 Cp = 环戊二烯基)通过 π 配位。在过去的几十年中,利用这些过渡金属单元的高吸电子性质和 η 6 -芳烃配合物的热力学稳定性,在结合芳烃上发生的反应的发展方面取得了显着的进展。一个典型的例子是将亲核芳族取代( SN Ar)反应扩展到电子中性和富卤代苯。此类芳烃通常对经典 S N Ar 呈惰性,可以经历涉及络合物形成、取代和络合物分解的序列。尽管通过利用预制配合物取得了成功,但可逆芳烃配位在催化系统中的应用仅取得有限的进展。因此,在π配位活化中,过渡金属单元通常被认为是结合芳烃配合物的组分,而不是π酸催化剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号