当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unraveling molecular mechanisms of β-glucuronidase inhibition by flavonoids from Centaurea scoparia: integrated in silico and in vitro insights
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2024-07-26 , DOI: 10.1039/d4nj02393e Maha A. Alwaili, Faris F. Aba Alkhayl, Hassan A. Rudayni, Ahmed A. Allam, Naif G. Altoom, Al Mokhtar Lamsabhi, Emadeldin M. Kamel
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2024-07-26 , DOI: 10.1039/d4nj02393e Maha A. Alwaili, Faris F. Aba Alkhayl, Hassan A. Rudayni, Ahmed A. Allam, Naif G. Altoom, Al Mokhtar Lamsabhi, Emadeldin M. Kamel
|
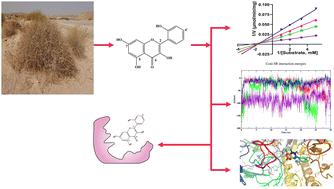
Investigating the detailed molecular mechanisms of β-glucuronidase inhibition is critical for pioneering new therapeutic solutions and driving progress in pharmaceutical research. The inhibitory potential of four flavonoid aglycones derived from Centaurea scoparia against β-glucuronidase was thoroughly examined using a combination of in vitro and in silico methodologies. The in vitro assays revealed that compounds 1 and 3 had the strongest inhibitory effects, demonstrated by their low IC50 values of 3.16 ± 0.34 and 3.82 ± 0.1 μM, respectively. The results of the enzyme kinetics assay revealed that compounds 2 and 3, and the reference drug EGCG displayed a mixed inhibition mode. Conversely, compound 1 was found to operate via a noncompetitive inhibition mechanism, as evidenced by the intersection patterns in the Lineweaver–Burk plots. The outcomes from the docking analysis are consistent with the in vitro inhibitory efficacy assays, showing that compounds 1 and 3 possess the lowest binding affinities (−8.6 and −9.0 kcal mol−1, respectively). Isolated phytochemicals demonstrated substantial polar and hydrophobic interactions with the residues within the enzyme's binding site. We investigated the interaction dynamics of isolated compounds with β-glucuronidase using a 100 ns molecular dynamics (MD) simulation. The analysis of various MD parameters indicated that compounds 1 and 3 showed stable trajectories and significant energy stabilization when bound to β-glucuronidase. Furthermore, compounds 1 and 3 exhibited the most favorable average Coulombic short-range interaction energies, recorded at −86.53 ± 11 kJ mol−1 and −98.04 ± 17 kJ mol−1, respectively. These compounds also demonstrated the lowest average Lennard–Jones short-range interaction energies, measured at −84.56 ± 14 kJ mol−1 and −106.02 ± 4.6 kJ mol−1, respectively. The results of MM/PBSA calculations revealed binding free energies of −10.99 ± 2.07, −11.39 ± 2.71, −26.77 ± 3.83, and 0.20 ± 0.32 kJ mol−1 for isolated compounds 1–4 with the target enzyme, respectively. These computational results support the experimental data, suggesting that compounds 1 and 3 could be potent inhibitors of β-glucuronidase.
中文翻译:

揭开矢车菊黄酮类化合物抑制 β-葡萄糖醛酸酶的分子机制:整合计算机和体外见解
研究 β-葡萄糖醛酸酶抑制的详细分子机制对于开拓新的治疗解决方案和推动药物研究的进展至关重要。使用体外和计算机方法相结合,彻底检查了源自矢车菊的四种黄酮苷元对 β-葡萄糖醛酸酶的抑制潜力。体外测定表明,化合物 1 和 3 具有最强的抑制作用,其 IC 50 值分别为 3.16 ± 0.34 和 3.82 ± 0.1 μM。酶动力学测定结果表明,化合物2和3以及参比药物EGCG表现出混合抑制模式。相反,我们发现化合物 1 通过非竞争性抑制机制发挥作用,如 Lineweaver-Burk 图中的交叉模式所证明的那样。对接分析的结果与体外抑制功效测定一致,表明化合物1和3具有最低的结合亲和力(分别为-8.6和-9.0 kcal mol −1 )。分离的植物化学物质显示出与酶结合位点内的残基存在大量的极性和疏水相互作用。我们使用 100 ns 分子动力学 (MD) 模拟研究了分离化合物与 β-葡萄糖醛酸酶的相互作用动力学。各种MD参数的分析表明,化合物1和3在与β-葡萄糖醛酸酶结合时表现出稳定的轨迹和显着的能量稳定性。此外,化合物1和3表现出最有利的平均库仑短程相互作用能,分别记录为-86.53±11kJmol −1 和-98.04±17kJmol −1 。 这些化合物还表现出最低的平均 Lennard-Jones 短程相互作用能,分别为 -84.56 ± 14 kJ mol −1 和 -106.02 ± 4.6 kJ mol −1 。 MM/PBSA 计算结果显示,分离的化合物 1-4 与目标酶的结合自由能为 -10.99 ± 2.07、-11.39 ± 2.71、-26.77 ± 3.83 和 0.20 ± 0.32 kJ mol −1 , 分别。这些计算结果支持了实验数据,表明化合物 1 和 3 可能是 β-葡萄糖醛酸酶的有效抑制剂。
更新日期:2024-07-26
中文翻译:

揭开矢车菊黄酮类化合物抑制 β-葡萄糖醛酸酶的分子机制:整合计算机和体外见解
研究 β-葡萄糖醛酸酶抑制的详细分子机制对于开拓新的治疗解决方案和推动药物研究的进展至关重要。使用体外和计算机方法相结合,彻底检查了源自矢车菊的四种黄酮苷元对 β-葡萄糖醛酸酶的抑制潜力。体外测定表明,化合物 1 和 3 具有最强的抑制作用,其 IC 50 值分别为 3.16 ± 0.34 和 3.82 ± 0.1 μM。酶动力学测定结果表明,化合物2和3以及参比药物EGCG表现出混合抑制模式。相反,我们发现化合物 1 通过非竞争性抑制机制发挥作用,如 Lineweaver-Burk 图中的交叉模式所证明的那样。对接分析的结果与体外抑制功效测定一致,表明化合物1和3具有最低的结合亲和力(分别为-8.6和-9.0 kcal mol −1 )。分离的植物化学物质显示出与酶结合位点内的残基存在大量的极性和疏水相互作用。我们使用 100 ns 分子动力学 (MD) 模拟研究了分离化合物与 β-葡萄糖醛酸酶的相互作用动力学。各种MD参数的分析表明,化合物1和3在与β-葡萄糖醛酸酶结合时表现出稳定的轨迹和显着的能量稳定性。此外,化合物1和3表现出最有利的平均库仑短程相互作用能,分别记录为-86.53±11kJmol −1 和-98.04±17kJmol −1 。 这些化合物还表现出最低的平均 Lennard-Jones 短程相互作用能,分别为 -84.56 ± 14 kJ mol −1 和 -106.02 ± 4.6 kJ mol −1 。 MM/PBSA 计算结果显示,分离的化合物 1-4 与目标酶的结合自由能为 -10.99 ± 2.07、-11.39 ± 2.71、-26.77 ± 3.83 和 0.20 ± 0.32 kJ mol −1 , 分别。这些计算结果支持了实验数据,表明化合物 1 和 3 可能是 β-葡萄糖醛酸酶的有效抑制剂。












































 京公网安备 11010802027423号
京公网安备 11010802027423号