当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ni(II) and Pd(II) complexes of a new redox-active pentadentate azo-appended 2-aminophenol ligand: Pd(II)-assisted intraligand cyclization forms a phenoxazinyl ring
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-07-24 , DOI: 10.1039/d4dt01513d Saumitra Bhowmik 1 , Arunava Sengupta 2 , Rabindranath Mukherjee 3
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-07-24 , DOI: 10.1039/d4dt01513d Saumitra Bhowmik 1 , Arunava Sengupta 2 , Rabindranath Mukherjee 3
Affiliation
|
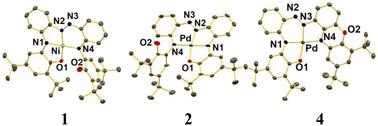
Square planar complexes of Ni(II) and Pd(II) of a new redox-active pentadentate azo-appended 2-aminophenol ligand (H4L = N,N′-bis(2-hydroxy-3,5-di-tert-butylphenyl)-2,2′-diamino-ortho-azobenzene) in three accessible redox levels [amidophenolate(2−), semiquinonate(1−) π radical, and quinone(0)] were synthesized. The coordinated HL(3−) ligand provides four donor sites [two N(iminophenolates), an N′(azo), and an O(phenolate)], while the phenolic OH group remains free in the three complexes. Cyclic voltammetry on complex [Ni(L)] 1 and its corresponding Pd(II) analogue [Pd(L)] 2 in CH2Cl2 displayed three redox responses (two oxidative at E1/2 = 0.06 V and Epa (anodic peak potential) = 0.80 V and one reductive at −0.77 V for 1 and at E1/2 = 0.08 V and Epa = 0.85 V and at −0.74 V for 2 vs. Fc+/Fc). The chemical oxidation of 1 with AgSbF6 afforded [Ni(L)]SbF6·2CH2Cl2 (3·2CH2Cl2). Complex [Pd(L*)] 4, which is coordinated by a phenoxazinyl derivative of L(4−), was obtained via intraligand cyclization in the parent complex 2 under basic oxidizing conditions. The molecular structures of 1, 2, 3·2CH2Cl2 and 4 were elucidated through X-ray crystallography at 100 K. Characterization using 1H NMR, X-band EPR, and UV–VIS–NIR spectroscopy established that the complexes have [NiII{(LISQ)˙2−}] 1, [PdII{(LISQ)˙2−}] 2, [NiII{(LIBQ)−}]SbF6/1+SbF6−(3), and [PdII{(L*AP)˙2−}] 4 electronic states. Complexes 1, 2, and 4 possess paramagnetic St (total spin) = 1/2 ground-state, whereas 3 is diamagnetic (St = 0). Density functional theory (DFT) electronic structural calculations at the B3LYP level rationalized the observed experimental results. Time-dependent (TD)-DFT calculations allowed us to identify the nature of the observed absorption spectra.
中文翻译:

新型氧化还原活性五齿偶氮连接的 2-氨基苯酚配体的 Ni(II) 和 Pd(II) 配合物:Pd(II) 辅助配体内环化形成吩恶嗪基环
新型氧化还原活性五齿偶氮连接的 2-氨基苯酚配体的 Ni( II ) 和 Pd( II ) 方形平面配合物 (H 4 L = N , N '-双(2-羟基-3,5-二叔)合成了三种可氧化还原水平的[氨基酚盐(2−)、半醌酸盐(1−)π自由基和醌(0)]。配位的 HL(3−) 配体提供四个供体位点 [两个 N(亚氨基酚盐)、一个 N'(偶氮)和一个 O(酚盐)],而酚 OH 基团在三个配合物中保持游离状态。 CH 2 Cl 2中配合物 [Ni(L)] 1及其相应的 Pd( II ) 类似物 [Pd(L)] 2的循环伏安法显示出三种氧化还原反应(两种氧化反应分别为E 1/2 = 0.06 V 和E pa (阳极峰值电位) = 0.80 V, 1为 -0.77 V, E 1/2 = 0.08 V 且E pa = 0.85 V, 2为 -0.74 V 时还原,相对于Fc + /Fc)。用AgSbF 6化学氧化1得到[Ni(L)]SbF 6 ·2CH 2 Cl 2 ( 3 ·2CH 2 Cl 2 )。 络合物[Pd(L*)] 4是由L(4-) 的吩恶嗪基衍生物配位的,是通过在碱性氧化条件下母体络合物2中的配体内环化获得的。通过 100 K 下的 X 射线晶体学阐明了1 , 2 , 3 ·2CH 2 Cl 2和4的分子结构。使用1 H NMR、X 波段 EPR 和 UV-VIS-NIR 光谱进行表征表明,这些配合物具有[Ni II {(L ISQ )˙ 2− }] 1 , [Pd II {(L ISQ )˙ 2− }] 2 , [Ni II {(L IBQ ) − }]SbF 6 / 1 + SbF 6 − ( 3 ) 和 [Pd II {(L* AP )˙ 2− }] 4 个电子态。 配合物1、2和4具有顺磁性St (总自旋)= 1/2 基态,而3是反磁性的( St = 0)。 B3LYP 水平的密度泛函理论 (DFT) 电子结构计算合理化了观察到的实验结果。时间相关 (TD)-DFT 计算使我们能够识别所观察到的吸收光谱的性质。
更新日期:2024-07-24
中文翻译:

新型氧化还原活性五齿偶氮连接的 2-氨基苯酚配体的 Ni(II) 和 Pd(II) 配合物:Pd(II) 辅助配体内环化形成吩恶嗪基环
新型氧化还原活性五齿偶氮连接的 2-氨基苯酚配体的 Ni( II ) 和 Pd( II ) 方形平面配合物 (H 4 L = N , N '-双(2-羟基-3,5-二叔)合成了三种可氧化还原水平的[氨基酚盐(2−)、半醌酸盐(1−)π自由基和醌(0)]。配位的 HL(3−) 配体提供四个供体位点 [两个 N(亚氨基酚盐)、一个 N'(偶氮)和一个 O(酚盐)],而酚 OH 基团在三个配合物中保持游离状态。 CH 2 Cl 2中配合物 [Ni(L)] 1及其相应的 Pd( II ) 类似物 [Pd(L)] 2的循环伏安法显示出三种氧化还原反应(两种氧化反应分别为E 1/2 = 0.06 V 和E pa (阳极峰值电位) = 0.80 V, 1为 -0.77 V, E 1/2 = 0.08 V 且E pa = 0.85 V, 2为 -0.74 V 时还原,相对于Fc + /Fc)。用AgSbF 6化学氧化1得到[Ni(L)]SbF 6 ·2CH 2 Cl 2 ( 3 ·2CH 2 Cl 2 )。 络合物[Pd(L*)] 4是由L(4-) 的吩恶嗪基衍生物配位的,是通过在碱性氧化条件下母体络合物2中的配体内环化获得的。通过 100 K 下的 X 射线晶体学阐明了1 , 2 , 3 ·2CH 2 Cl 2和4的分子结构。使用1 H NMR、X 波段 EPR 和 UV-VIS-NIR 光谱进行表征表明,这些配合物具有[Ni II {(L ISQ )˙ 2− }] 1 , [Pd II {(L ISQ )˙ 2− }] 2 , [Ni II {(L IBQ ) − }]SbF 6 / 1 + SbF 6 − ( 3 ) 和 [Pd II {(L* AP )˙ 2− }] 4 个电子态。 配合物1、2和4具有顺磁性St (总自旋)= 1/2 基态,而3是反磁性的( St = 0)。 B3LYP 水平的密度泛函理论 (DFT) 电子结构计算合理化了观察到的实验结果。时间相关 (TD)-DFT 计算使我们能够识别所观察到的吸收光谱的性质。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号