当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decrypting the hydrogen evolution in alkaline water with novel magnetoactive cobalt(II) complex-driven cobalt oxide electrocatalysts
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-07-24 , DOI: 10.1039/d4dt01358a Subhajit Saha 1 , Nilankar Diyali 1 , Sangharaj Diyali 1 , Subhra Jyoti Panda 2 , Mainak Das 3 , Sobhna Acharya 4 , Prafullya Kumar Mudi 5 , Monika Singh 4 , Partha Pratim Ray 3 , Chandra Shekhar Purohit 2 , Bhaskar Biswas 1
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-07-24 , DOI: 10.1039/d4dt01358a Subhajit Saha 1 , Nilankar Diyali 1 , Sangharaj Diyali 1 , Subhra Jyoti Panda 2 , Mainak Das 3 , Sobhna Acharya 4 , Prafullya Kumar Mudi 5 , Monika Singh 4 , Partha Pratim Ray 3 , Chandra Shekhar Purohit 2 , Bhaskar Biswas 1
Affiliation
|
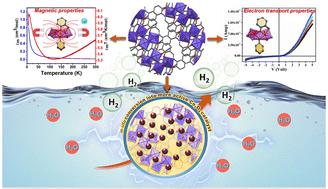
Under the gravity of future socio-economic development, the viability of water electrolysis still hinges on the accessibility of stable earth-abundant electrocatalysts and net energy efficiency. This work emphasizes the design and synthesis of two newly developed cobalt(II) complexes, [Co(HL)2(NCS)2] (Comono) and [Co2(L)3(CH3OH)]ClO4 (Codi), with a (N,O)-donor ligand, HL (2-methoxy-6-(((2-methoxyphenyl)imino)methyl)phenol). The study delves into understanding their structural, morphological, magnetic, and charge transport characteristics. Moreover, the study explores the potential of these complexes in catalyzing hydrogen production through heterogeneous electrocatalysis. The X-ray crystal structure of Comono reveals the octahedral geometry of the Co(II) ion, adopting two HL units and two NCS− units. The Codi complex exhibits a doubly-phenoxo-O-bridged (μ1,1) dinuclear complex, forming a typical octahedral geometry for both the Co(II) centres in coupling with three units of L−. Temperature-dependent magnetic susceptibility measurements showed that all of the Co(II) ion in Comono shows a typical paramagnetic behaviour for high spin octahedral Co(II) ions while the Co(II) centres in Codi are coupled with doubly-phenoxo-bridges bearing weak ferromagnetic characteristics at low temperature. Electron transport properties of the Co(II) complex-mediated Schottky device address the superior carrier mobility (μ) for Codi (9.21 × 10−5) over Comono (2.02 × 10−5 m2 v−1 s−1) with respective transit times of 1.70 × 10−9 and 7.77 × 10−9 s. Additionally, electron impedance spectral analysis supports the lower electrical transport resistance of Codi relative to Comono. The heterogeneous electrocatalytic HER activity of Codi and Comono in 0.1 M KOH shows excellent electrocatalytic efficiency in terms of the various electrochemical parameters. Constant potential electrolysis, multi-cycle CVs, and post-HER analysis reveal the pre-catalytic nature of the complexes, which in turn delivers Co3O4 nanoparticles as the active catalysts for efficient hydrogen evolution.
中文翻译:

用新型磁活性钴(II)络合物驱动的氧化钴电催化剂解密碱性水中的析氢
在未来社会经济发展的重力下,水电解的可行性仍然取决于能否获得稳定的地球丰富的电催化剂和净能源效率。这项工作重点介绍了两种新开发的钴( II )配合物的设计和合成,即[Co(H L ) 2 (NCS) 2 ] ( Co mono )和[Co 2 ( L ) 3 (CH 3 OH)]ClO 4 ( Co di ),具有 (N,O)-供体配体 H L (2-甲氧基-6-(((2-甲氧基苯基)亚氨基)甲基)苯酚)。该研究深入了解它们的结构、形态、磁性和电荷传输特性。此外,该研究还探讨了这些配合物通过非均相电催化催化制氢的潜力。 Co mono的 X 射线晶体结构揭示了 Co( II ) 离子的八面体几何结构,采用两个 HL 单元和两个 NCS -单元。 Co di配合物呈现出双苯氧基-O-桥接 (μ 1,1 ) 双核配合物,两个 Co( II ) 中心与三个L -单元耦合形成典型的八面体几何形状。 与温度相关的磁化率测量表明, Co mono中的所有 Co( II ) 离子均表现出高自旋八面体 Co( II ) 离子的典型顺磁行为,而Co di中的 Co( II ) 中心与双苯氧偶合。桥梁在低温下具有弱铁磁特性。 Co( II )络合物介导的肖特基器件的电子传输特性解决了Co di (9.21 × 10 -5 )优于Co mono (2.02 × 10 -5 m 2 v -1 s -1 )的载流子迁移率( μ )的问题其渡越时间分别为1.70 × 10 -9和7.77 × 10 -9 s。此外,电子阻抗谱分析支持Co di相对于Co mono具有较低的电传输电阻。 Co di和Co mono在 0.1 M KOH 中的非均相电催化 HER 活性在各种电化学参数方面表现出优异的电催化效率。 恒电位电解、多循环CV和HER后分析揭示了配合物的预催化性质,这反过来又提供Co 3 O 4纳米颗粒作为有效析氢的活性催化剂。
更新日期:2024-07-24
中文翻译:

用新型磁活性钴(II)络合物驱动的氧化钴电催化剂解密碱性水中的析氢
在未来社会经济发展的重力下,水电解的可行性仍然取决于能否获得稳定的地球丰富的电催化剂和净能源效率。这项工作重点介绍了两种新开发的钴( II )配合物的设计和合成,即[Co(H L ) 2 (NCS) 2 ] ( Co mono )和[Co 2 ( L ) 3 (CH 3 OH)]ClO 4 ( Co di ),具有 (N,O)-供体配体 H L (2-甲氧基-6-(((2-甲氧基苯基)亚氨基)甲基)苯酚)。该研究深入了解它们的结构、形态、磁性和电荷传输特性。此外,该研究还探讨了这些配合物通过非均相电催化催化制氢的潜力。 Co mono的 X 射线晶体结构揭示了 Co( II ) 离子的八面体几何结构,采用两个 HL 单元和两个 NCS -单元。 Co di配合物呈现出双苯氧基-O-桥接 (μ 1,1 ) 双核配合物,两个 Co( II ) 中心与三个L -单元耦合形成典型的八面体几何形状。 与温度相关的磁化率测量表明, Co mono中的所有 Co( II ) 离子均表现出高自旋八面体 Co( II ) 离子的典型顺磁行为,而Co di中的 Co( II ) 中心与双苯氧偶合。桥梁在低温下具有弱铁磁特性。 Co( II )络合物介导的肖特基器件的电子传输特性解决了Co di (9.21 × 10 -5 )优于Co mono (2.02 × 10 -5 m 2 v -1 s -1 )的载流子迁移率( μ )的问题其渡越时间分别为1.70 × 10 -9和7.77 × 10 -9 s。此外,电子阻抗谱分析支持Co di相对于Co mono具有较低的电传输电阻。 Co di和Co mono在 0.1 M KOH 中的非均相电催化 HER 活性在各种电化学参数方面表现出优异的电催化效率。 恒电位电解、多循环CV和HER后分析揭示了配合物的预催化性质,这反过来又提供Co 3 O 4纳米颗粒作为有效析氢的活性催化剂。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号