当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synergy of experimental and computational chemistry: structure and biological activity of Zn(II) hydrazone complexes
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-07-23 , DOI: 10.1039/d4dt01353k Milica Savić , Andrej Pevec , Nevena Stevanović , Irena Novaković , Ivana Matic , Nina Petrovic , Tatjana Stanojkovic , Karla Milčić , Matija Zlatar , Iztok Turel , Božidar Čobeljić , Milos Milcic , Maja Gruden
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-07-23 , DOI: 10.1039/d4dt01353k Milica Savić , Andrej Pevec , Nevena Stevanović , Irena Novaković , Ivana Matic , Nina Petrovic , Tatjana Stanojkovic , Karla Milčić , Matija Zlatar , Iztok Turel , Božidar Čobeljić , Milos Milcic , Maja Gruden
|
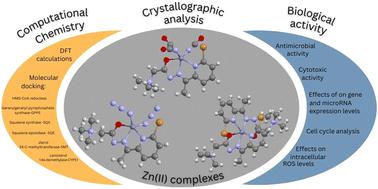
In this paper, three different Zn(II) complexes with (E)-2-(2-(1-(6-bromopyridin-2-yl)ethylidene)hydrazinyl)-N,N,N-trimethyl-2-oxoethan-1-aminium chloride (HLCl) have been synthesized and characterized by single crystal X-ray diffraction, elemental analysis, IR and NMR spectroscopy. All complexes are mononuclear, with the ligand (L) coordinated in a deprotonated formally neutral zwitterionic form via NNO donor set atoms. Complex 1 forms an octahedral geometry with the composition [ZnL2](BF4)2, while complexes 2 [ZnL(NCO)2] and 3 [ZnL(N3)2] form penta-coordinated geometry. Density functional theory (DFT) calculations were performed to enhance our understanding of the structures of the synthesized complexes and the cytotoxic activity of the complexes was tested against five human cancer cell lines (HeLa, A549, MDA-MB-231, K562, LS 174T) and normal human fibroblasts MRC-5. Additionally, antibacterial and antifungal activity of these complexes was tested against a panel of Gram-negative and Gram-positive bacteria, two fungal strains, and a yeast strain. It is noteworthy that all three complexes show selective antifungal activity comparable to that of amphotericin B. Molecular docking analysis predicted that geranylgeranyl pyrophosphate synthase, an enzyme essential for sterol biosynthesis, is the most likely target for inhibition by the tested complexes.
中文翻译:

实验和计算化学的协同作用:Zn(II)腙配合物的结构和生物活性
在本文中,三种不同的 Zn(II) 与 (E)-2-(2-(1-(6-溴吡啶-2-基)亚乙基)肼基)-N,N,N-三甲基-2-氧代乙烷-合成了 1-氯化铵 (HLCl),并通过单晶 X 射线衍射、元素分析、红外和核磁共振光谱进行了表征。所有配合物都是单核的,配体 (L) 通过 NNO 供体原子以去质子化的形式中性两性离子形式配位。配合物 1 形成八面体几何形状,其组成为 [ZnL 2 ](BF 4 ) 2 ,而配合物 2 [ZnL(NCO) 2 ) 2 ] 形成五配位几何。进行密度泛函理论 (DFT) 计算以增强我们对合成复合物结构的理解,并针对五种人类癌细胞系(HeLa、A549、MDA-MB-231、K562、LS 174T)测试复合物的细胞毒活性)和正常人成纤维细胞MRC-5。此外,还针对一组革兰氏阴性和革兰氏阳性细菌、两种真菌菌株和一种酵母菌株测试了这些复合物的抗菌和抗真菌活性。值得注意的是,所有三种复合物都显示出与两性霉素 B 相当的选择性抗真菌活性。分子对接分析预测,香叶基香叶基焦磷酸合酶(甾醇生物合成所必需的酶)是所测试复合物最有可能的抑制靶点。
更新日期:2024-07-26
中文翻译:

实验和计算化学的协同作用:Zn(II)腙配合物的结构和生物活性
在本文中,三种不同的 Zn(II) 与 (E)-2-(2-(1-(6-溴吡啶-2-基)亚乙基)肼基)-N,N,N-三甲基-2-氧代乙烷-合成了 1-氯化铵 (HLCl),并通过单晶 X 射线衍射、元素分析、红外和核磁共振光谱进行了表征。所有配合物都是单核的,配体 (L) 通过 NNO 供体原子以去质子化的形式中性两性离子形式配位。配合物 1 形成八面体几何形状,其组成为 [ZnL 2 ](BF 4 ) 2 ,而配合物 2 [ZnL(NCO) 2 ) 2 ] 形成五配位几何。进行密度泛函理论 (DFT) 计算以增强我们对合成复合物结构的理解,并针对五种人类癌细胞系(HeLa、A549、MDA-MB-231、K562、LS 174T)测试复合物的细胞毒活性)和正常人成纤维细胞MRC-5。此外,还针对一组革兰氏阴性和革兰氏阳性细菌、两种真菌菌株和一种酵母菌株测试了这些复合物的抗菌和抗真菌活性。值得注意的是,所有三种复合物都显示出与两性霉素 B 相当的选择性抗真菌活性。分子对接分析预测,香叶基香叶基焦磷酸合酶(甾醇生物合成所必需的酶)是所测试复合物最有可能的抑制靶点。












































 京公网安备 11010802027423号
京公网安备 11010802027423号