Acta Neuropathologica ( IF 9.3 ) Pub Date : 2024-07-24 , DOI: 10.1007/s00401-024-02774-2 Gannon A McDonough 1 , Yuchen Cheng 2, 3 , Katherine S Morillo 2 , Ryan N Doan 2, 4 , Zinan Zhou 2 , Connor J Kenny 2 , Aaron Foutz 5 , Chae Kim 5, 6 , Mark L Cohen 5, 6, 7 , Brian S Appleby 5, 6, 7 , Christopher A Walsh 2, 4, 8 , Jiri G Safar 5, 6, 9 , August Yue Huang 2, 4 , Michael B Miller 1, 2, 4
|
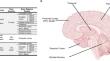
Creutzfeldt–Jakob Disease (CJD), the most common human prion disease, is associated with pathologic misfolding of the prion protein (PrP), encoded by the PRNP gene. Of human prion disease cases, < 1% were transmitted by misfolded PrP, ~ 15% are inherited, and ~ 85% are sporadic (sCJD). While familial cases are inherited through germline mutations in PRNP, the cause of sCJD is unknown. Somatic mutations have been hypothesized as a cause of sCJD, and recent studies have revealed that somatic mutations accumulate in neurons during aging. To investigate the hypothesis that somatic mutations in PRNP may underlie sCJD, we performed deep DNA sequencing of PRNP in 205 sCJD cases and 170 age-matched non-disease controls. We included 5 cases of Heidenhain variant sporadic CJD (H-sCJD), where visual symptomatology and neuropathology implicate localized initiation of prion formation, and examined multiple regions across the brain including in the affected occipital cortex. We employed Multiple Independent Primer PCR Sequencing (MIPP-Seq) with a median depth of > 5000× across the PRNP coding region and analyzed for variants using MosaicHunter. An allele mixing experiment showed positive detection of variants in bulk DNA at a variant allele fraction (VAF) as low as 0.2%. We observed multiple polymorphic germline variants among individuals in our cohort. However, we did not identify bona fide somatic variants in sCJD, including across multiple affected regions in H-sCJD, nor in control individuals. Beyond our stringent variant-identification pipeline, we also analyzed VAFs from raw sequencing data, and observed no evidence of prion disease enrichment for the known germline pathogenic variants P102L, D178N, and E200K. The lack of PRNP pathogenic somatic mutations in H-sCJD or the broader cohort of sCJD suggests that clonal somatic mutations may not play a major role in sporadic prion disease. With H-sCJD representing a localized presentation of neurodegeneration, this serves as a test of the potential role of clonal somatic mutations in genes known to cause familial neurodegeneration.
中文翻译:

散发性人类朊病毒病中 PRNP 体细胞和种系变异的神经病理学指导分析
克雅氏病 (CJD) 是最常见的人类朊病毒病,与PRNP基因编码的朊病毒蛋白 (PrP) 的病理性错误折叠有关。在人类朊病毒病病例中,< 1% 是通过错误折叠的 PrP 传播的,约 15% 是遗传性的,约 85% 是散发性的 (sCJD)。虽然家族性病例是通过PRNP种系突变遗传的,但 sCJD 的病因尚不清楚。体细胞突变被认为是导致 sCJD 的原因,最近的研究表明,体细胞突变在衰老过程中在神经元中积累。为了研究PRNP体细胞突变可能是 sCJD 的基础这一假设,我们对 205 名 sCJD 病例和 170 名年龄匹配的非疾病对照者进行了PRNP深度 DNA 测序。我们纳入了 5 例海德汉变异型散发性克雅氏病 (H-sCJD) 病例,其中视觉症状学和神经病理学表明朊病毒形成的局部起始,并检查了大脑的多个区域,包括受影响的枕叶皮质。我们采用多重独立引物 PCR 测序 (MIPP-Seq), PRNP编码区域的中位深度 > 5000×,并使用 MosaicHunter 分析变异。等位基因混合实验显示,在大量 DNA 中,阳性检测到变异等位基因分数 (VAF) 低至 0.2%。我们在我们的队列中的个体中观察到多种多态性种系变异。然而,我们没有在 sCJD 中发现真正的体细胞变异,包括在 H-sCJD 的多个受影响区域,也没有在对照个体中发现。除了我们严格的变异识别流程之外,我们还分析了原始测序数据中的 VAF,没有观察到已知种系致病变异 P102L、D178N 和 E200K 的朊病毒疾病富集的证据。 H-sCJD 或更广泛的 sCJD 群体中缺乏PRNP致病性体细胞突变,这表明克隆体细胞突变可能在散发性朊病毒病中不起主要作用。 H-sCJD 代表神经变性的局部表现,这可以作为已知导致家族性神经变性的基因中克隆体细胞突变的潜在作用的测试。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号