Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of an eco-friendly capillary electrophoresis method for the simultaneous determination of piperacillin, tazobactam and ibuprofen in plasma samples: application to a pharmacokinetic study in rats
RSC Advances ( IF 3.9 ) Pub Date : 2024-07-24 , DOI: 10.1039/d4ra04615c Weam M Othman 1 , Nourah Z Al-Zoman 2 , Ibrahim A Darwish 2 , Aliyah Almomen 2 , Samah S Saad 1 , Fatma F Abdallah 3 , Nehal F Farid 3
RSC Advances ( IF 3.9 ) Pub Date : 2024-07-24 , DOI: 10.1039/d4ra04615c Weam M Othman 1 , Nourah Z Al-Zoman 2 , Ibrahim A Darwish 2 , Aliyah Almomen 2 , Samah S Saad 1 , Fatma F Abdallah 3 , Nehal F Farid 3
Affiliation
|
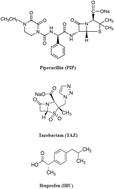
Piperacillin (PIP) and tazobactam (TAZ) are broad-spectrum beta-lactam antimicrobial agents, which are frequently co-prescribed in intensive care units (ICUs) worldwide. Ibuprofen (IBU) is a potent pain killer which is commonly co-prescribed with PIP and TAZ postoperatively. The combination therapy of PIP, TAZ, and IBU has been administered commonly after surgical procedures to combat aerobic and anaerobic microbes and exert anti-inflammatory and analgesic effects. This study describes, for the first time, the development of a new capillary electrophoresis (CE) method with a photodiode array detector for the simultaneous determination of PIP, TAZ, and IBU in plasma samples. The experimental factors affecting the elution of analytes were carefully optimized. The final analysis was achieved using a fused silica capillary (58 cm effective length and 75 μm ID) and a background electrolyte solution containing a methanol/borax buffer solution (15 mM and pH 9.3) in a ratio of (10 : 90 v/v) with a driving voltage of 30 kV and detection at 210 nm. The relationship between the peak area and concentration was linear from 1 to 200 μg mL−1 for both PIP and TAZ and from 3 to 200 μg mL−1 for TAZ. The method used was thoroughly validated in accordance with the validation requirements set out by the Food and Drug Administration (FDA) for bio-analytical processes. The proposed CE method was employed to conduct pharmacokinetic and bioavailability studies of the drugs in rat models. The pharmacokinetic results revealed that there is a significant impact upon prescribing this combination concurrently when compared to their single administration. To illustrate, the time required to reach their maximum concentrations (Tmax) was increased by 0.25 h for both PIP and TAZ, whereas it was increased by 0.5 for IBU. When it comes to their maximum concentration (Cmax), it was increased by 13.7%, 55.5%, and 44% for PIP, TAZ, and IBU, respectively. Furthermore, the bioavailabilities of PIP, TAZ, and IBU were significantly increased by 55.4%, 19.7%, and 35.6%, respectively. These findings require caution when these drugs are co-prescribed as there is a noticeable augmentation in their therapeutic impacts. Additionally, the greenness of the proposed method was assessed by three metric tools. In conclusion, the method is a valuable tool for further studies on drug–drug interaction in humans.
中文翻译:

开发一种同时测定血浆样品中哌拉西林、他唑巴坦和布洛芬的环保毛细管电泳方法:在大鼠药代动力学研究中的应用
哌拉西林 (PIP) 和他唑巴坦 (TAZ) 是广谱 β-内酰胺类抗菌药物,在世界各地的重症监护病房 (ICU) 中经常联合使用。布洛芬 (IBU) 是一种强效止痛药,通常在术后与 PIP 和 TAZ 联合使用。 PIP、TAZ和IBU联合治疗常用于外科手术后,以对抗需氧和厌氧微生物,发挥抗炎和镇痛作用。这项研究首次描述了一种新的毛细管电泳 (CE) 方法的开发,该方法采用光电二极管阵列检测器,用于同时测定血浆样品中的 PIP、TAZ 和 IBU。影响分析物洗脱的实验因素经过仔细优化。最终分析是使用熔融石英毛细管(58 cm 有效长度和 75 μm ID)和含有比例为(10 : 90 v/v)的甲醇/硼砂缓冲溶液(15 mM 和 pH 9.3)的背景电解质溶液来实现的。 ),驱动电压为 30 kV,检测波长为 210 nm。 PIP 和 TAZ 的峰面积与浓度之间的关系在 1 至 200 μg mL -1范围内呈线性关系,而 TAZ 则在 3 至 200 μg mL -1范围内呈线性关系。所使用的方法根据美国食品和药物管理局 (FDA) 为生物分析过程制定的验证要求进行了彻底验证。采用所提出的CE方法在大鼠模型中进行药物的药代动力学和生物利用度研究。药代动力学结果表明,与单次给药相比,同时开出该组合药物具有显着影响。 为了说明这一点,PIP 和 TAZ 达到最大浓度 ( T max ) 所需的时间增加了 0.25 小时,而 IBU 则增加了 0.5 小时。当达到最大浓度( C max )时,PIP、TAZ 和 IBU 分别增加了 13.7%、55.5% 和 44%。此外,PIP、TAZ 和 IBU 的生物利用度分别显着增加 55.4%、19.7% 和 35.6%。当这些药物共同处方时,需要谨慎对待这些发现,因为它们的治疗效果明显增强。此外,所提出方法的绿色性通过三种度量工具进行了评估。总之,该方法是进一步研究人类药物相互作用的宝贵工具。
更新日期:2024-07-24
中文翻译:

开发一种同时测定血浆样品中哌拉西林、他唑巴坦和布洛芬的环保毛细管电泳方法:在大鼠药代动力学研究中的应用
哌拉西林 (PIP) 和他唑巴坦 (TAZ) 是广谱 β-内酰胺类抗菌药物,在世界各地的重症监护病房 (ICU) 中经常联合使用。布洛芬 (IBU) 是一种强效止痛药,通常在术后与 PIP 和 TAZ 联合使用。 PIP、TAZ和IBU联合治疗常用于外科手术后,以对抗需氧和厌氧微生物,发挥抗炎和镇痛作用。这项研究首次描述了一种新的毛细管电泳 (CE) 方法的开发,该方法采用光电二极管阵列检测器,用于同时测定血浆样品中的 PIP、TAZ 和 IBU。影响分析物洗脱的实验因素经过仔细优化。最终分析是使用熔融石英毛细管(58 cm 有效长度和 75 μm ID)和含有比例为(10 : 90 v/v)的甲醇/硼砂缓冲溶液(15 mM 和 pH 9.3)的背景电解质溶液来实现的。 ),驱动电压为 30 kV,检测波长为 210 nm。 PIP 和 TAZ 的峰面积与浓度之间的关系在 1 至 200 μg mL -1范围内呈线性关系,而 TAZ 则在 3 至 200 μg mL -1范围内呈线性关系。所使用的方法根据美国食品和药物管理局 (FDA) 为生物分析过程制定的验证要求进行了彻底验证。采用所提出的CE方法在大鼠模型中进行药物的药代动力学和生物利用度研究。药代动力学结果表明,与单次给药相比,同时开出该组合药物具有显着影响。 为了说明这一点,PIP 和 TAZ 达到最大浓度 ( T max ) 所需的时间增加了 0.25 小时,而 IBU 则增加了 0.5 小时。当达到最大浓度( C max )时,PIP、TAZ 和 IBU 分别增加了 13.7%、55.5% 和 44%。此外,PIP、TAZ 和 IBU 的生物利用度分别显着增加 55.4%、19.7% 和 35.6%。当这些药物共同处方时,需要谨慎对待这些发现,因为它们的治疗效果明显增强。此外,所提出方法的绿色性通过三种度量工具进行了评估。总之,该方法是进一步研究人类药物相互作用的宝贵工具。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号