当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphine-catalyzed [4 + 3] and [4 + 4] annulations of β′-acetoxy allenoates with N,N-dinucleophiles: access to 1,3-diazepine and 1,4-diazocine derivatives
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-24 , DOI: 10.1039/d4qo00949e Chunjie Ni 1 , Zhanhang Liang 1 , Xiaojuan Xu 1 , Fan Yu 1 , Yining Zhao 1 , Chen Chen 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-24 , DOI: 10.1039/d4qo00949e Chunjie Ni 1 , Zhanhang Liang 1 , Xiaojuan Xu 1 , Fan Yu 1 , Yining Zhao 1 , Chen Chen 2
Affiliation
|
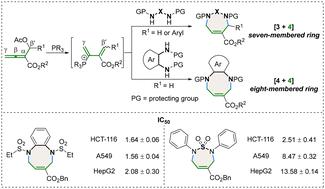
Novel methods for constructing functionalized 1,3-diazepine and 1,4-diazocine derivatives have been disclosed through phosphine-catalyzed [4 + 3] and [4 + 4] annulations of β′-acetoxy allenoates with N,N-dinucleophiles. These approaches demonstrate high efficiency and yields ranging from good to excellent. The biological evaluation indicates that three of the developed cycloadducts exhibit satisfactory inhibitory activities against three human cancer cell lines (HCT116, A549 and HepG2) beyond those of 5-fluorouracil.
中文翻译:

膦催化的 β'-乙酰氧基联烯酸酯与 N,N-二亲核试剂的 [4 + 3] 和 [4 + 4] 环化:获得 1,3-二氮杂卓和 1,4-二氮杂辛衍生物
已经公开了通过β'-乙酰氧基联烯酸酯与N , N-二亲核试剂的膦催化[4+3]和[4+4]环化来构建官能化1,3-二氮杂卓和1,4-二氮杂辛衍生物的新方法。这些方法表现出高效率和从良好到优秀的产量。生物学评价表明,所开发的三种环加合物对三种人类癌细胞系(HCT116、A549和HepG2)表现出优于5-氟尿嘧啶的令人满意的抑制活性。
更新日期:2024-07-29
中文翻译:

膦催化的 β'-乙酰氧基联烯酸酯与 N,N-二亲核试剂的 [4 + 3] 和 [4 + 4] 环化:获得 1,3-二氮杂卓和 1,4-二氮杂辛衍生物
已经公开了通过β'-乙酰氧基联烯酸酯与N , N-二亲核试剂的膦催化[4+3]和[4+4]环化来构建官能化1,3-二氮杂卓和1,4-二氮杂辛衍生物的新方法。这些方法表现出高效率和从良好到优秀的产量。生物学评价表明,所开发的三种环加合物对三种人类癌细胞系(HCT116、A549和HepG2)表现出优于5-氟尿嘧啶的令人满意的抑制活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号