当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Ligand-Enabled Cyclization of Substituted Aliphatic Carboxylic Acids with Allylic Electrophiles
Organic Letters ( IF 4.9 ) Pub Date : 2024-07-25 , DOI: 10.1021/acs.orglett.4c02129 Gouranga Naskar 1 , Masilamani Jeganmohan 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-07-25 , DOI: 10.1021/acs.orglett.4c02129 Gouranga Naskar 1 , Masilamani Jeganmohan 1
Affiliation
|
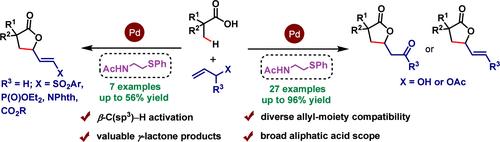
A palladium-catalyzed cyclization of the β-C(sp3)–H bond of aliphatic carboxylic acids with allylic electrophiles providing five-membered γ-lactones in good to excellent yields is demonstrated. An acetyl-protected aminoethyl phenyl thioether ligand is used to promote the C–H activation reaction. A diverse range of allylic electrophiles such as allyl alcohols, allyl acetates, allyl sulfones, allyl phosphonate, allyl amine, and allyl ester have been utilized for this reaction. A feasible reaction mechanism has been proposed to account for the present cyclization reaction.
中文翻译:

钯催化的配体使取代的脂肪族羧酸与烯丙基亲电子试剂发生环化
钯催化的脂肪族羧酸的 β-C(sp 3 )–H 键与烯丙基亲电子试剂的环化反应可以以良好至优异的产率提供五元 γ-内酯。乙酰基保护的氨基乙基苯基硫醚配体用于促进 C-H 活化反应。该反应已使用多种烯丙基亲电子试剂,例如烯丙醇、乙酸烯丙酯、烯丙基砜、膦酸烯丙酯、烯丙胺和烯丙酯。提出了一种可行的反应机制来解释目前的环化反应。
更新日期:2024-07-25
中文翻译:

钯催化的配体使取代的脂肪族羧酸与烯丙基亲电子试剂发生环化
钯催化的脂肪族羧酸的 β-C(sp 3 )–H 键与烯丙基亲电子试剂的环化反应可以以良好至优异的产率提供五元 γ-内酯。乙酰基保护的氨基乙基苯基硫醚配体用于促进 C-H 活化反应。该反应已使用多种烯丙基亲电子试剂,例如烯丙醇、乙酸烯丙酯、烯丙基砜、膦酸烯丙酯、烯丙胺和烯丙酯。提出了一种可行的反应机制来解释目前的环化反应。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号