当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Double Spirocyclization of 2-Benzyl-3-alkynyl Chromone with Nitrone via Gold-Catalyzed Cascade Reactions
Organic Letters ( IF 4.9 ) Pub Date : 2024-07-25 , DOI: 10.1021/acs.orglett.4c02338 Amit Vijay Sasane, Chun-Tang Chiou, Ming-Yiang Chang, Wen-Tai Li
Organic Letters ( IF 4.9 ) Pub Date : 2024-07-25 , DOI: 10.1021/acs.orglett.4c02338 Amit Vijay Sasane, Chun-Tang Chiou, Ming-Yiang Chang, Wen-Tai Li
|
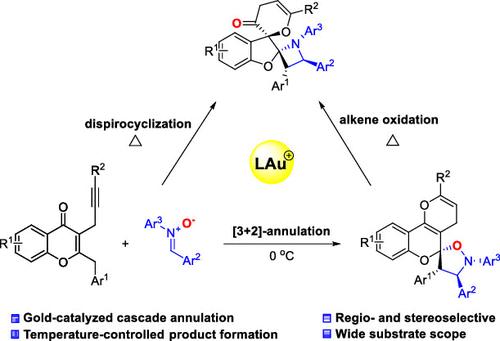
A novel, highly stereoselective gold-catalyzed spirocyclization of 2-benzyl-3-alkynyl chromone with nitrone is described. This cascade reaction involves gold-catalyzed cycloisomerization, nitrone-olefin [3 + 2]-annulation, alkene oxidation, and rearrangement for the formation of spirocyclic products. Interestingly, the isoxazolidine ring generated from [3 + 2]-annulation donates oxygen to alkene to generate a new pyran-3(4H)-one and azetidine ring for dispiro-benzofuran formation upon heating. This work demonstrates the one-pot, gold-catalyzed, multiple-step reaction, and the reaction temperature directly affects the formation of spirocyclic products.
中文翻译:

通过金催化级联反应进行 2-苄基-3-炔基色酮与硝酮的立体选择性双螺环化
描述了一种新型的、高度立体选择性的金催化 2-苄基-3-炔基色酮与硝酮的螺环化反应。该级联反应涉及金催化的环异构化、硝酮-烯烃[3+2]-成环、烯烃氧化以及形成螺环产物的重排。有趣的是,由[3 + 2]-环化生成的异恶唑烷环将氧提供给烯烃,生成新的吡喃-3(4 H )-酮和氮杂环丁烷环,在加热时形成二螺-苯并呋喃。这项工作展示了金催化的一锅多步反应,反应温度直接影响螺环产物的形成。
更新日期:2024-07-25
中文翻译:

通过金催化级联反应进行 2-苄基-3-炔基色酮与硝酮的立体选择性双螺环化
描述了一种新型的、高度立体选择性的金催化 2-苄基-3-炔基色酮与硝酮的螺环化反应。该级联反应涉及金催化的环异构化、硝酮-烯烃[3+2]-成环、烯烃氧化以及形成螺环产物的重排。有趣的是,由[3 + 2]-环化生成的异恶唑烷环将氧提供给烯烃,生成新的吡喃-3(4 H )-酮和氮杂环丁烷环,在加热时形成二螺-苯并呋喃。这项工作展示了金催化的一锅多步反应,反应温度直接影响螺环产物的形成。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号