当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Alkoxide-Assisted Stereoselective Functionalization of 1,2-Bis-boronic Esters Under Photoredox Catalysis
Organic Letters ( IF 4.9 ) Pub Date : 2024-07-25 , DOI: 10.1021/acs.orglett.4c02469 Somenath Mahato 1 , Debraj Ghorai 1 , Kanak Kanti Das 1 , Lisa Roy 2 , Santanu Panda 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-07-25 , DOI: 10.1021/acs.orglett.4c02469 Somenath Mahato 1 , Debraj Ghorai 1 , Kanak Kanti Das 1 , Lisa Roy 2 , Santanu Panda 1
Affiliation
|
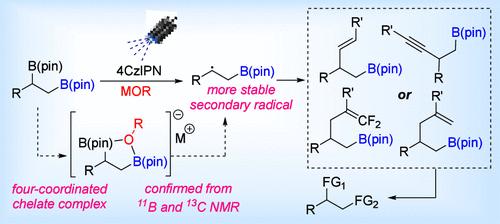
Site-specific functionalization of the secondary C–B bond of 1,2-bis-boronic esters has been proven to be an important method for the generation of 1,2-bis-functionalized compounds in a highly stereoselective manner. We have explored previously unknown secondary selective alkenylation, allylation, alkynylation and addition to aryl vinyl trifluoromethane, which proceeds via a novel reaction mechanism: alkoxide-mediated photoredox activation to generate secondary radicals over the primary one.
中文翻译:

光氧化还原催化下醇盐辅助的 1,2-双硼酸酯立体选择性官能化
1,2-双硼酸酯的仲C-B键的位点特异性官能化已被证明是以高度立体选择性的方式生成1,2-双官能化化合物的重要方法。我们探索了以前未知的二级选择性烯基化、烯丙基化、炔基化和对芳基乙烯基三氟甲烷的加成,其通过一种新的反应机制进行:醇盐介导的光氧化还原活化以在初级自由基上产生二级自由基。
更新日期:2024-07-26
中文翻译:

光氧化还原催化下醇盐辅助的 1,2-双硼酸酯立体选择性官能化
1,2-双硼酸酯的仲C-B键的位点特异性官能化已被证明是以高度立体选择性的方式生成1,2-双官能化化合物的重要方法。我们探索了以前未知的二级选择性烯基化、烯丙基化、炔基化和对芳基乙烯基三氟甲烷的加成,其通过一种新的反应机制进行:醇盐介导的光氧化还原活化以在初级自由基上产生二级自由基。












































 京公网安备 11010802027423号
京公网安备 11010802027423号