当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
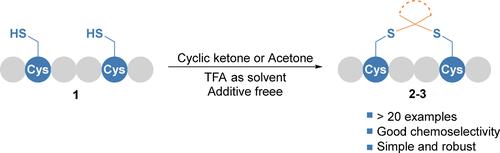
Trifluoroacetic Acid Mediated Additive-Free Late-Stage Native Peptide Cyclization to Form Disulfide Mimetics via Thioketalization with Ketones
Organic Letters ( IF 4.9 ) Pub Date : 2024-07-24 , DOI: 10.1021/acs.orglett.4c02464 Yisa Xiao 1 , Haiyan Zhou 1, 2 , Han Liu 1 , Xuechen Li 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-07-24 , DOI: 10.1021/acs.orglett.4c02464 Yisa Xiao 1 , Haiyan Zhou 1, 2 , Han Liu 1 , Xuechen Li 1
Affiliation
|
Peptide cyclization is often used to introduce conformational rigidity and to enhance the physiological stability of the peptide. This study presents a novel late-stage cyclization method for creating thioketal cyclic peptides from bis-cysteine peptides and drugs. Symmetrical cyclic ketones and acetone were found to react with bis-cysteine unprotected peptides efficiently to form thioketal linkages in trifluoroacetic acid (TFA) without any other additive. The attractive features of this method include high chemoselectivity, operational simplicity, and robustness. In addition, TFA as the reaction solvent can dissolve any unprotected peptide. As a showcase, the dimethyl thioketal versions of lanreotide and octreotide were prepared and evaluated, both of which showed much improved reductive stability and comparable activity.
中文翻译:

三氟乙酸介导的无添加剂后期天然肽环化通过酮硫酮化形成二硫键模拟物
肽环化通常用于引入构象刚性并增强肽的生理稳定性。这项研究提出了一种新的后期环化方法,用于从双半胱氨酸肽和药物产生硫缩酮环肽。研究发现,对称环酮和丙酮可与双半胱氨酸未保护的肽有效反应,在三氟乙酸 (TFA) 中形成硫酮键,无需任何其他添加剂。该方法的吸引人的特点包括高化学选择性、操作简单性和鲁棒性。此外,TFA作为反应溶剂可以溶解任何未保护的肽。作为展示,制备并评估了兰瑞肽和奥曲肽的二甲基硫缩酮版本,两者都显示出大大改善的还原稳定性和相当的活性。
更新日期:2024-07-26
中文翻译:

三氟乙酸介导的无添加剂后期天然肽环化通过酮硫酮化形成二硫键模拟物
肽环化通常用于引入构象刚性并增强肽的生理稳定性。这项研究提出了一种新的后期环化方法,用于从双半胱氨酸肽和药物产生硫缩酮环肽。研究发现,对称环酮和丙酮可与双半胱氨酸未保护的肽有效反应,在三氟乙酸 (TFA) 中形成硫酮键,无需任何其他添加剂。该方法的吸引人的特点包括高化学选择性、操作简单性和鲁棒性。此外,TFA作为反应溶剂可以溶解任何未保护的肽。作为展示,制备并评估了兰瑞肽和奥曲肽的二甲基硫缩酮版本,两者都显示出大大改善的还原稳定性和相当的活性。












































 京公网安备 11010802027423号
京公网安备 11010802027423号