当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microfluidic-Derived Docosahexaenoic Acid Liposomes for Targeting Glioblastoma and Its Inflammatory Microenvironment
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-07-23 , DOI: 10.1021/acsami.4c01368 Daniel Mendanha 1, 2 , Marta R Casanova 1, 2 , Sara Gimondi 1, 2 , Helena Ferreira 1, 2 , Nuno M Neves 1, 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-07-23 , DOI: 10.1021/acsami.4c01368 Daniel Mendanha 1, 2 , Marta R Casanova 1, 2 , Sara Gimondi 1, 2 , Helena Ferreira 1, 2 , Nuno M Neves 1, 2
Affiliation
|
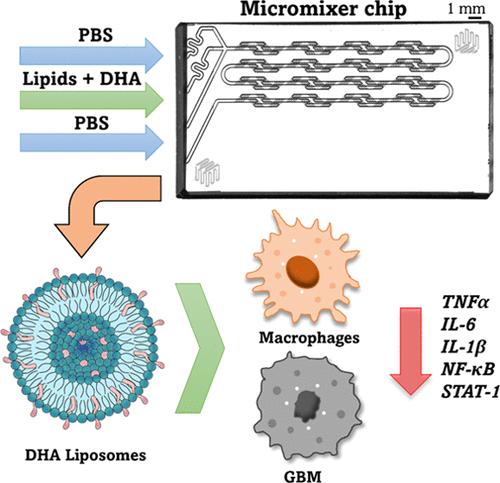
Glioblastoma (GBM) is the most common malignant primary brain tumor, characterized by limited treatment options and a poor prognosis. Its aggressiveness is attributed not only to the uncontrolled proliferation and invasion of tumor cells but also to the complex interplay between these cells and the surrounding microenvironment. Within the tumor microenvironment, an intricate network of immune cells, stromal cells, and various signaling molecules creates a pro-inflammatory milieu that supports tumor growth and progression. Docosahexaenoic acid (DHA), an essential ω3 polyunsaturated fatty acid for brain function, is associated with anti-inflammatory and anticarcinogenic properties. Therefore, in this work, DHA liposomes were synthesized using a microfluidic platform to target and reduce the inflammatory environment of GBM. The liposomes were rapidly taken up by macrophages in a time-dependent manner without causing cytotoxicity. Moreover, DHA liposomes successfully downregulated the expression of inflammatory-associated genes (IL-6; IL-1β; TNFα; NF-κB, and STAT-1) and the secretion of key cytokines (IL-6 and TNFα) in stimulated macrophages and GBM cells. Conversely, no significant differences were observed in the expression of IL-10, an anti-inflammatory gene expressed in alternatively activated macrophages. Additionally, DHA liposomes were found to be more efficient in regulating the inflammatory profile of these cells compared with a free formulation of DHA. The nanomedicine platform established in this work opens new opportunities for developing liposomes incorporating DHA to target GBM and its inflammatory milieu.
中文翻译:

微流体衍生的二十二碳六烯酸脂质体用于靶向胶质母细胞瘤及其炎症微环境
胶质母细胞瘤(GBM)是最常见的恶性原发性脑肿瘤,其特点是治疗选择有限且预后不良。其侵袭性不仅归因于肿瘤细胞不受控制的增殖和侵袭,还归因于这些细胞与周围微环境之间复杂的相互作用。在肿瘤微环境中,免疫细胞、基质细胞和各种信号分子的复杂网络创造了支持肿瘤生长和进展的促炎环境。二十二碳六烯酸 (DHA) 是大脑功能必需的 ω3 多不饱和脂肪酸,具有抗炎和抗癌特性。因此,在这项工作中,利用微流体平台合成DHA脂质体来靶向和减少GBM的炎症环境。脂质体以时间依赖性方式被巨噬细胞快速吸收,而不会引起细胞毒性。此外,DHA脂质体成功下调受刺激的巨噬细胞和炎症相关基因( IL-6 、 IL-1β 、 TNFα 、 NF-κB和STAT-1 )的表达以及关键细胞因子(IL-6和TNFα)的分泌。 GBM细胞。相反, IL-10 (一种在交替激活的巨噬细胞中表达的抗炎基因)的表达没有观察到显着差异。此外,与游离 DHA 制剂相比,DHA 脂质体在调节这些细胞的炎症方面更有效。这项工作中建立的纳米医学平台为开发含有 DHA 的脂质体以靶向 GBM 及其炎症环境开辟了新的机会。
更新日期:2024-07-23
中文翻译:

微流体衍生的二十二碳六烯酸脂质体用于靶向胶质母细胞瘤及其炎症微环境
胶质母细胞瘤(GBM)是最常见的恶性原发性脑肿瘤,其特点是治疗选择有限且预后不良。其侵袭性不仅归因于肿瘤细胞不受控制的增殖和侵袭,还归因于这些细胞与周围微环境之间复杂的相互作用。在肿瘤微环境中,免疫细胞、基质细胞和各种信号分子的复杂网络创造了支持肿瘤生长和进展的促炎环境。二十二碳六烯酸 (DHA) 是大脑功能必需的 ω3 多不饱和脂肪酸,具有抗炎和抗癌特性。因此,在这项工作中,利用微流体平台合成DHA脂质体来靶向和减少GBM的炎症环境。脂质体以时间依赖性方式被巨噬细胞快速吸收,而不会引起细胞毒性。此外,DHA脂质体成功下调受刺激的巨噬细胞和炎症相关基因( IL-6 、 IL-1β 、 TNFα 、 NF-κB和STAT-1 )的表达以及关键细胞因子(IL-6和TNFα)的分泌。 GBM细胞。相反, IL-10 (一种在交替激活的巨噬细胞中表达的抗炎基因)的表达没有观察到显着差异。此外,与游离 DHA 制剂相比,DHA 脂质体在调节这些细胞的炎症方面更有效。这项工作中建立的纳米医学平台为开发含有 DHA 的脂质体以靶向 GBM 及其炎症环境开辟了新的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号