当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Controlling DNA Fragments Translocation across Nanopores with the Synergic Use of Site-Directed Mutagenesis, pH-Dependent Charge Tuning, and Electroosmotic Flow
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-07-22 , DOI: 10.1021/acsami.4c03848 Loredana Mereuta 1 , Huma Bhatti 2 , Alina Asandei 3 , Adina Cimpanu 1 , Yi-Lun Ying 2 , Yi-Tao Long 2 , Tudor Luchian 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-07-22 , DOI: 10.1021/acsami.4c03848 Loredana Mereuta 1 , Huma Bhatti 2 , Alina Asandei 3 , Adina Cimpanu 1 , Yi-Lun Ying 2 , Yi-Tao Long 2 , Tudor Luchian 1
Affiliation
|
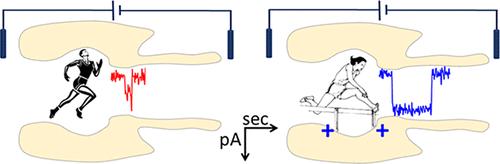
Biological and solid-state nanopores are at the core of transformative techniques and nanodevices, democratizing the examination of matter and biochemical reactions at the single-molecule level, with low cost, portability, and simplicity in operation. One of the crucial hurdles in such endeavors is the fast analyte translocation, which limits characterization, and a rich number of strategies have been explored over the years to overcome this. Here, by site-directed mutagenesis on the α-hemolysin protein nanopore (α-HL), sought to replace selected amino acids with glycine, electrostatic binding sites were induced on the nanopore’s vestibule and constriction region and achieved in the most favorable case a 20-fold increase in the translocation time of short single-stranded DNA (ssDNA) at neutral pH, with respect to the wild-type (WT) nanopore. We demonstrated an efficient tool of controlling the ssDNA translocation time, via the interplay between the nanopore-ssDNA surface electrostatic interactions and electroosmotic flow, all mediated by the pH-dependent ionization of amino acids lining the nanopore’s translocation pathway. Our data also reveal the nonmonotonic, pH-induced alteration of ssDNA average translocation time. Unlike mildly acidic conditions (pH ∼ 4.7), at a pH ∼ 2.8 maintained symmetrically or asymmetrically across the WT α-HL, we evidenced the manifestation of a dominant electroosmotic flow, determining the speeding up of the ssDNA translocation across the nanopore by counteracting the ssDNA-nanopore attractive electrostatic interactions. We envision potential applications of the presented approach by enabling easy-to-use, real-time detection of short ssDNA sequences, without the need for complex biochemical modifications to the nanopore to mitigate the fast translocation of such sequences.
中文翻译:

通过协同使用定点诱变、pH 依赖性电荷调节和电渗流来控制 DNA 片段跨纳米孔的易位
生物和固态纳米孔是变革技术和纳米器件的核心,使单分子水平上的物质和生化反应的检查民主化,且成本低、便携且操作简单。这种努力的关键障碍之一是分析物的快速易位,这限制了表征,多年来已经探索了多种策略来克服这一问题。在这里,通过对 α-溶血素蛋白纳米孔 (α-HL) 进行定点诱变,试图用甘氨酸替换选定的氨基酸,在纳米孔的前庭和收缩区域上诱导静电结合位点,并在最有利的情况下实现了 20 - 相对于野生型 (WT) 纳米孔,短单链 DNA (ssDNA) 在中性 pH 下的易位时间增加了一倍。我们展示了一种控制单链DNA易位时间的有效工具,通过纳米孔-单链DNA表面静电相互作用和电渗流之间的相互作用,所有这些都是由纳米孔易位途径内的氨基酸的pH依赖性电离介导的。我们的数据还揭示了 ssDNA 平均易位时间的非单调、pH 诱导的变化。与弱酸性条件(pH ∼ 4.7)不同,在 WT α-HL 上保持对称或不对称的 pH ∼ 2.8 下,我们证明了主导电渗流的表现,通过抵消ssDNA-纳米孔吸引静电相互作用。 我们设想了所提出方法的潜在应用,即能够易于使用、实时检测短单链DNA序列,而不需要对纳米孔进行复杂的生化修饰来减轻此类序列的快速易位。
更新日期:2024-07-24
中文翻译:

通过协同使用定点诱变、pH 依赖性电荷调节和电渗流来控制 DNA 片段跨纳米孔的易位
生物和固态纳米孔是变革技术和纳米器件的核心,使单分子水平上的物质和生化反应的检查民主化,且成本低、便携且操作简单。这种努力的关键障碍之一是分析物的快速易位,这限制了表征,多年来已经探索了多种策略来克服这一问题。在这里,通过对 α-溶血素蛋白纳米孔 (α-HL) 进行定点诱变,试图用甘氨酸替换选定的氨基酸,在纳米孔的前庭和收缩区域上诱导静电结合位点,并在最有利的情况下实现了 20 - 相对于野生型 (WT) 纳米孔,短单链 DNA (ssDNA) 在中性 pH 下的易位时间增加了一倍。我们展示了一种控制单链DNA易位时间的有效工具,通过纳米孔-单链DNA表面静电相互作用和电渗流之间的相互作用,所有这些都是由纳米孔易位途径内的氨基酸的pH依赖性电离介导的。我们的数据还揭示了 ssDNA 平均易位时间的非单调、pH 诱导的变化。与弱酸性条件(pH ∼ 4.7)不同,在 WT α-HL 上保持对称或不对称的 pH ∼ 2.8 下,我们证明了主导电渗流的表现,通过抵消ssDNA-纳米孔吸引静电相互作用。 我们设想了所提出方法的潜在应用,即能够易于使用、实时检测短单链DNA序列,而不需要对纳米孔进行复杂的生化修饰来减轻此类序列的快速易位。












































 京公网安备 11010802027423号
京公网安备 11010802027423号