当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Preparation of Neptunyl and Plutonyl Acetates To Access Nonaqueous Transuranium Coordination Chemistry
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-24 , DOI: 10.1021/jacs.4c04613 Emily R. Mikeska 1, 2 , Richard E. Wilson 2 , Asmita Sen 3 , Jochen Autschbach 3 , James D. Blakemore 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-24 , DOI: 10.1021/jacs.4c04613 Emily R. Mikeska 1, 2 , Richard E. Wilson 2 , Asmita Sen 3 , Jochen Autschbach 3 , James D. Blakemore 1
Affiliation
|
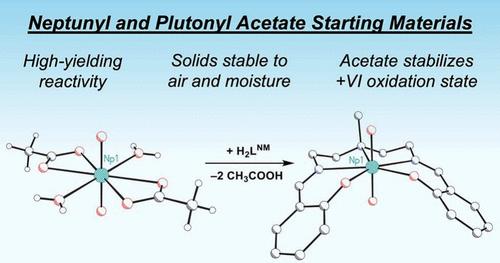
Uranyl diacetate dihydrate is a useful reagent for the preparation of uranyl (UO22+) coordination complexes, as it is a well-defined stoichiometric compound featuring moderately basic acetates that can facilitate protonolysis reactivity, unlike other anions commonly used in synthetic actinide chemistry such as halides or nitrate. Despite these attractive features, analogous neptunium (Np) and plutonium (Pu) compounds are unknown to date. Here, a modular synthetic route is reported for accessing stoichiometric neptunyl(VI) and plutonyl(VI) diacetate compounds that can serve as starting materials for transuranic coordination chemistry. The new NpO22+ and PuO22+ complexes, as well as a corresponding molecular UO22+ complex, are isomorphous in the solid state, and in solution show similar solubility properties that facilitate their use in synthesis. In both solid and solution state, the +VI oxidation state (O.S.) is maintained, as demonstrated by vibrational and optical spectroscopy, confirming that acetate anions stabilize the oxidizing, high-valent +VI states of Np and Pu as they do for the more stable U(VI). All three acetate salts readily react with a model diprotic ligand, affording incorporation of U(VI), Np(VI), and Pu(VI) cores into molecular coordination compounds that occurs concomitantly with elimination of acetic acid; the new complexes are high-valent, yet overall charge neutral, facilitating entry into nonaqueous chemistry by rational synthesis. Computational studies reveal that the dianionic ligand framework assists in stabilizing the +VI O.S. via donation to the 5f shells of the actinides, highlighting the potential usefulness of protonolysis reactivity toward preparation of stabilized high-valent transuranic species.
中文翻译:

乙酸庚烷酯和乙酸钚酰酯的制备以获取非水超铀配位化学
二乙酸铀酰二水合物是一种用于制备铀酰 (UO 2 2+ ) 配位配合物的有用试剂,因为它是一种明确的化学计量化合物,具有中等碱性的乙酸盐,可以促进质子解反应活性,与合成锕系化学中常用的其他阴离子(例如卤化物或硝酸根)不同。尽管具有这些吸引人的特征,但类似的镎 (Np) 和钚 (Pu) 化合物迄今为止仍未知。在此,报道了一种模块化合成路线,用于获取化学计量的庚烯基 (VI) 和钍酰 (VI) 二乙酸酯化合物,这些化合物可用作超铀配位化学的起始材料。新的NpO 2 2+ 和PuO 2 2+ 配合物,以及相应的分子UO 2 < b7> 络合物在固态下是同晶的,并且在溶液中表现出相似的溶解度特性,这有利于它们在合成中的使用。在固体和溶液状态下,如振动和光谱所证明的,+VI 氧化态 (O.S.) 得以维持,证实乙酸根阴离子稳定了 Np 和 Pu 的氧化性高价 +VI 态,就像它们在更多情况下所做的那样。稳定的U(VI)。所有三种乙酸盐都很容易与模型二元配体反应,将 U(VI)、Np(VI) 和 Pu(VI) 核心掺入分子配位化合物中,同时消除乙酸;新的配合物是高价的,但总体呈中性,有助于通过合理合成进入非水化学领域。计算研究表明,双阴离子配体框架有助于稳定 +VI O.S. 通过捐赠给锕系元素的 5f 壳,强调了质子分解反应对于制备稳定的高价超铀物种的潜在用途。
更新日期:2024-07-25
中文翻译:

乙酸庚烷酯和乙酸钚酰酯的制备以获取非水超铀配位化学
二乙酸铀酰二水合物是一种用于制备铀酰 (UO 2 2+ ) 配位配合物的有用试剂,因为它是一种明确的化学计量化合物,具有中等碱性的乙酸盐,可以促进质子解反应活性,与合成锕系化学中常用的其他阴离子(例如卤化物或硝酸根)不同。尽管具有这些吸引人的特征,但类似的镎 (Np) 和钚 (Pu) 化合物迄今为止仍未知。在此,报道了一种模块化合成路线,用于获取化学计量的庚烯基 (VI) 和钍酰 (VI) 二乙酸酯化合物,这些化合物可用作超铀配位化学的起始材料。新的NpO 2 2+ 和PuO 2 2+ 配合物,以及相应的分子UO 2 < b7> 络合物在固态下是同晶的,并且在溶液中表现出相似的溶解度特性,这有利于它们在合成中的使用。在固体和溶液状态下,如振动和光谱所证明的,+VI 氧化态 (O.S.) 得以维持,证实乙酸根阴离子稳定了 Np 和 Pu 的氧化性高价 +VI 态,就像它们在更多情况下所做的那样。稳定的U(VI)。所有三种乙酸盐都很容易与模型二元配体反应,将 U(VI)、Np(VI) 和 Pu(VI) 核心掺入分子配位化合物中,同时消除乙酸;新的配合物是高价的,但总体呈中性,有助于通过合理合成进入非水化学领域。计算研究表明,双阴离子配体框架有助于稳定 +VI O.S. 通过捐赠给锕系元素的 5f 壳,强调了质子分解反应对于制备稳定的高价超铀物种的潜在用途。












































 京公网安备 11010802027423号
京公网安备 11010802027423号